当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Virtual Plant for Integrated Continuous Manufacturing of a Carfilzomib Drug Substance Intermediate, Part 2: Enone Synthesis via a Barbier-Type Grignard Process
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-14 , DOI: 10.1021/acs.oprd.0c00188 Elçin Içten 1 , Andrew J. Maloney 2 , Matthew G. Beaver 1 , Xiaoxiang Zhu 1 , Dongying E. Shen 1 , Jo Anna Robinson 1 , Andrew T. Parsons 1 , Ayman Allian 3 , Seth Huggins 3 , Roger Hart 1 , Pablo Rolandi 1 , Shawn D. Walker 1 , Richard D. Braatz 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-14 , DOI: 10.1021/acs.oprd.0c00188 Elçin Içten 1 , Andrew J. Maloney 2 , Matthew G. Beaver 1 , Xiaoxiang Zhu 1 , Dongying E. Shen 1 , Jo Anna Robinson 1 , Andrew T. Parsons 1 , Ayman Allian 3 , Seth Huggins 3 , Roger Hart 1 , Pablo Rolandi 1 , Shawn D. Walker 1 , Richard D. Braatz 2
Affiliation
|
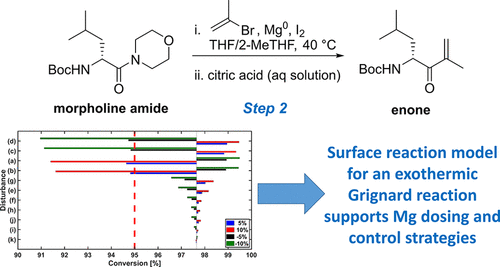
This article details efforts to characterize and develop a process control strategy for the manufacture of enone 2, a carfilzomib drug substance intermediate obtained through a Barbier-type Grignard reaction of morpholine amide 1. This includes the development of a novel mechanistic model for the heterogeneous Barbier-type Grignard reaction. After the model was characterized with laboratory-scale batch experiments, its performance was compared with experimental data collected under continuous operating conditions. Under nominal operating conditions, the experimentally measured conversion of morpholine amide varied from 94.3% to 96.7%, a range that was encompassed by the model. With a mechanistic model validated under continuous operating conditions, relationships between the magnesium charging interval and the variability in conversion of morpholine amide 1 to enone 2 were determined to further explore the experimental design space. The remaining unit operations were subsequently characterized, and the models developed for the individual operations were integrated into a flowsheet-level dynamic process model implemented in the gPROMS FormulatedProducts software. The impact of various process disturbances and model uncertainties on the critical quality attributes were then investigated, and critical process parameters, failure modes, and control strategies to address these disturbances were identified. The process was found to be most sensitive to operational disturbances in the supplied reactants: morpholine amide 1 and 2-bromopropene (2-BP). As 1 is manufactured upstream by the process described in Part 1 of this series, in silico analysis of potential process control strategies focused on manipulation of the 2-BP concentration and flow rate into the primary reactor. Overall, this work highlights the benefits of using mathematical modeling to deepen the understanding of pharmaceutical manufacturing processes and enable integrated unit operations in a continuous manufacturing setting.
中文翻译:

用于连续连续生产卡非佐米药物中间体的虚拟工厂,第2部分:通过Barbier型格氏试剂合成烯酮
本文详细介绍了表征和开发工艺控制策略以制造烯酮2的过程,烯酮2是通过吗啉酰胺1的Barbier型格氏反应获得的卡非佐米原料药中间体。。这包括为异质Barbier型格氏反应建立新的机理模型。在通过实验室规模的批处理实验对模型进行表征后,将其性能与在连续操作条件下收集的实验数据进行了比较。在标称操作条件下,实验测得的吗啉酰胺转化率从94.3%到96.7%不等,该范围已被模型涵盖。通过在连续操作条件下验证的机理模型,镁的充电间隔与吗啉酰胺1转化为烯酮2的转化率之间的关系决心进一步探索实验设计空间。随后对其余的单元操作进行了表征,并将针对单个操作开发的模型集成到了在gPROMS FormulatedProducts软件中实现的流程图级动态过程模型中。然后研究了各种过程干扰和模型不确定性对关键质量属性的影响,并确定了解决这些干扰的关键过程参数,故障模式和控制策略。发现该方法对所提供的反应物:吗啉酰胺1和2-溴丙烯(2-BP)的操作干扰最敏感。作为1通过本系列第1部分中描述的方法在上游制造SPC,对潜在的过程控制策略进行计算机分析,重点是控制2-BP浓度和进入主反应器的流速。总的来说,这项工作强调了使用数学建模来加深对制药生产过程的理解并在连续生产环境中实现集成单元操作的好处。
更新日期:2020-07-14
中文翻译:

用于连续连续生产卡非佐米药物中间体的虚拟工厂,第2部分:通过Barbier型格氏试剂合成烯酮
本文详细介绍了表征和开发工艺控制策略以制造烯酮2的过程,烯酮2是通过吗啉酰胺1的Barbier型格氏反应获得的卡非佐米原料药中间体。。这包括为异质Barbier型格氏反应建立新的机理模型。在通过实验室规模的批处理实验对模型进行表征后,将其性能与在连续操作条件下收集的实验数据进行了比较。在标称操作条件下,实验测得的吗啉酰胺转化率从94.3%到96.7%不等,该范围已被模型涵盖。通过在连续操作条件下验证的机理模型,镁的充电间隔与吗啉酰胺1转化为烯酮2的转化率之间的关系决心进一步探索实验设计空间。随后对其余的单元操作进行了表征,并将针对单个操作开发的模型集成到了在gPROMS FormulatedProducts软件中实现的流程图级动态过程模型中。然后研究了各种过程干扰和模型不确定性对关键质量属性的影响,并确定了解决这些干扰的关键过程参数,故障模式和控制策略。发现该方法对所提供的反应物:吗啉酰胺1和2-溴丙烯(2-BP)的操作干扰最敏感。作为1通过本系列第1部分中描述的方法在上游制造SPC,对潜在的过程控制策略进行计算机分析,重点是控制2-BP浓度和进入主反应器的流速。总的来说,这项工作强调了使用数学建模来加深对制药生产过程的理解并在连续生产环境中实现集成单元操作的好处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号