当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Virtual Plant for Integrated Continuous Manufacturing of a Carfilzomib Drug Substance Intermediate, Part 3: Manganese-Catalyzed Asymmetric Epoxidation, Crystallization, and Filtration
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-14 , DOI: 10.1021/acs.oprd.0c00189 Andrew J. Maloney 1 , Elçin Içten 2 , Gerard Capellades 1 , Matthew G. Beaver 2 , Xiaoxiang Zhu 2 , Lauren R. Graham 2 , Derek B. Brown 3 , Daniel J. Griffin 2 , Rahul Sangodkar 2 , Ayman Allian 3 , Seth Huggins 3 , Roger Hart 2 , Pablo Rolandi 2 , Shawn D. Walker 2 , Richard D. Braatz 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-14 , DOI: 10.1021/acs.oprd.0c00189 Andrew J. Maloney 1 , Elçin Içten 2 , Gerard Capellades 1 , Matthew G. Beaver 2 , Xiaoxiang Zhu 2 , Lauren R. Graham 2 , Derek B. Brown 3 , Daniel J. Griffin 2 , Rahul Sangodkar 2 , Ayman Allian 3 , Seth Huggins 3 , Roger Hart 2 , Pablo Rolandi 2 , Shawn D. Walker 2 , Richard D. Braatz 1
Affiliation
|
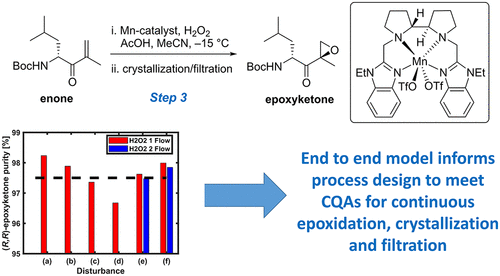
This article describes the process characterization and development of models to inform a process control strategy to prepare (R,R)-epoxy ketone 2, an intermediate in the manufacture of carfilzomib. Model calibration for relevant unit operations and the development of a dynamic integrated flowsheet-level model in gPROMS FormulatedProducts software enabled investigation of the impact of process disturbances and model uncertainties on the critical quality attributes (CQAs) and identification of critical process disturbances and failure modes to guide a process control strategy. The model development was similar to that described in the previous parts of this series, but with the added complexity of comparing two distinct kinetic formulations for the epoxidation reaction. The main CQAs for this process were (1) the conversion of enone 1 (target ≥99.0 mol % conversion) and (2) the purity target for solids prior to cake wash (target ≥97.5% purity by weight). Conversion of enone was not always achieved with the expected disturbances: whereas 99.5% conversion was expected for normal operating conditions, 97.2% conversion was predicted for the worst-case combination of disturbances. The chiral purity of crystalline (R,R)-epoxy ketone 2 was not always achieved with the expected disturbances: 98.2% purity was expected for normal operating conditions, and 96.7% purity was expected for the worst-case combination of disturbances. These analyses allowed for rank ordering of critical process parameters that impact conversion and suitable manipulated variables to develop a robust process control strategy for the manufacturing scheme.
中文翻译:

连续连续生产Carfilzomib药物中间体的虚拟工厂,第3部分:锰催化的不对称环氧化,结晶和过滤
本文介绍了过程表征和模型开发过程,以告知制备(R,R)-环氧酮2的过程控制策略,是卡非佐米生产中的中间体。在gPROMS FormulatedProducts软件中对相关单元操作进行模型校准以及开发动态集成的流程级模型,从而能够研究过程干扰和模型不确定性对关键质量属性(CQA)的影响,并识别出关键的过程干扰和故障模式,指导过程控制策略。该模型的开发与本系列前面部分中描述的模型开发类似,但是比较环氧化反应的两种不同动力学配方的方法增加了复杂性。此过程的主要CQA是(1)烯酮1的转化(目标≥99.0mol%转化率)和(2)滤饼洗涤前固体的纯度目标(目标纯度≥97.5重量%)。烯酮的转化并非总是能在预期的干扰条件下实现的:在正常工作条件下,转化率有望达到99.5%,对于最坏的干扰组合,预测转化率为97.2%。结晶(R,R)-环氧酮2的手性纯度。并非总是能在预期的干扰条件下实现:在正常工作条件下,预期纯度为98.2%,在最坏的情况下,预期纯度为96.7%。这些分析允许对影响转化的关键过程参数和合适的受控变量进行排序,从而为制造方案开发出可靠的过程控制策略。
更新日期:2020-07-14
中文翻译:

连续连续生产Carfilzomib药物中间体的虚拟工厂,第3部分:锰催化的不对称环氧化,结晶和过滤
本文介绍了过程表征和模型开发过程,以告知制备(R,R)-环氧酮2的过程控制策略,是卡非佐米生产中的中间体。在gPROMS FormulatedProducts软件中对相关单元操作进行模型校准以及开发动态集成的流程级模型,从而能够研究过程干扰和模型不确定性对关键质量属性(CQA)的影响,并识别出关键的过程干扰和故障模式,指导过程控制策略。该模型的开发与本系列前面部分中描述的模型开发类似,但是比较环氧化反应的两种不同动力学配方的方法增加了复杂性。此过程的主要CQA是(1)烯酮1的转化(目标≥99.0mol%转化率)和(2)滤饼洗涤前固体的纯度目标(目标纯度≥97.5重量%)。烯酮的转化并非总是能在预期的干扰条件下实现的:在正常工作条件下,转化率有望达到99.5%,对于最坏的干扰组合,预测转化率为97.2%。结晶(R,R)-环氧酮2的手性纯度。并非总是能在预期的干扰条件下实现:在正常工作条件下,预期纯度为98.2%,在最坏的情况下,预期纯度为96.7%。这些分析允许对影响转化的关键过程参数和合适的受控变量进行排序,从而为制造方案开发出可靠的过程控制策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号