当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adding a Gastric Step to the Intestinal In Vitro Digestion Model Improves the Prediction of Pharmacokinetic Data in Beagle Dogs of Two Lipid-Based Drug Delivery Systems.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-14 , DOI: 10.1021/acs.molpharmaceut.0c00307 Mette Klitgaard 1 , Stephane Beilles 2 , Philip Jonas Sassene 1 , Ragna Berthelsen 1 , Anette Müllertz 1, 3
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-14 , DOI: 10.1021/acs.molpharmaceut.0c00307 Mette Klitgaard 1 , Stephane Beilles 2 , Philip Jonas Sassene 1 , Ragna Berthelsen 1 , Anette Müllertz 1, 3
Affiliation
|
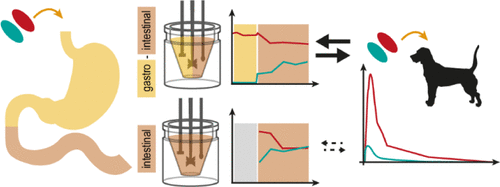
Drug release from a lipid-based drug delivery system (LbDDS) is typically studied in vitro using a one-step intestinal digestion model. However, lately the importance of incorporating gastric digestion has been stressed. The aim of the present study was to compare a two-step gastro-intestinal (GI) in vitro digestion model to the commonly used one-step intestinal digestion model. The models were evaluated by studying release of the model drug A1260 from two LbDDSs (F-I and F-II), for which in vivo pharmacokinetic data from oral administration to beagle dogs were available. The amount of A1260 recovered in the aqueous phases during and after the GI digestion of F-I and F-II was related to the Cmax and AUC0–48h of the plasma concentration–time profiles of each formulation and produced a rank order in vitro–in vivo (IVIV) relation. In comparison, a similar IVIV rank ordering was obtained when relating the amount of A1260 recovered in the aqueous phase prior (t = 0 min), and following 15 min of intestinal digestion, to the plasma concentration–time profiles. However, after 60 min of intestinal digestion, the LbDDSs performed equally in the one-step in vitro digestion model, contrary to what was observed in the two-step digestion model, and in vivo. As the GI digestion model produced a clearer distinction in terms of LbDDS rank ordering of the two LbDDSs, compared to the intestinal digestion model, it was found to be a promising in vitro model to study and estimate the LbDDS behavior in vivo.
中文翻译:

在肠道体外消化模型中增加一个胃步骤,可以改善两种基于脂质的药物递送系统的比格犬中药代动力学数据的预测。
通常使用一步肠消化模型在体外研究基于脂质的药物递送系统(LbDDS)的药物释放。但是,最近强调了结合胃消化的重要性。本研究的目的是将两步式胃肠(GI)体外消化模型与常用的一步式肠消化模型进行比较。通过研究模型药物A1260从两种LbDDS(FI和F-II)中的释放来评估模型,对于这些药物,可以得到从口服给药到比格犬的体内药代动力学数据。GI消化FI和F-II期间和之后,在水相中回收的A1260的量与C max和AUC 0-48h有关每种制剂的血浆浓度-时间分布曲线,并在体外-体内(IVIV)关系中产生了等级顺序。相比之下,将肠道消化前(t = 0分钟)和消化15分钟后在水相中回收的A1260的量与血浆浓度-时间曲线相关时,可获得类似的IVIV等级排序。但是,在肠道消化60分钟后,LbDDS在一步一步体外消化模型中的表现均与在两步消化模型中观察到的相反,并且在体内。与肠道消化模型相比,由于GI消化模型在两种LbDDS的LbDDS等级排序方面产生了更清晰的区别,因此发现它是研究和评估体内LbDDS行为的有希望的体外模型。
更新日期:2020-09-09
中文翻译:

在肠道体外消化模型中增加一个胃步骤,可以改善两种基于脂质的药物递送系统的比格犬中药代动力学数据的预测。
通常使用一步肠消化模型在体外研究基于脂质的药物递送系统(LbDDS)的药物释放。但是,最近强调了结合胃消化的重要性。本研究的目的是将两步式胃肠(GI)体外消化模型与常用的一步式肠消化模型进行比较。通过研究模型药物A1260从两种LbDDS(FI和F-II)中的释放来评估模型,对于这些药物,可以得到从口服给药到比格犬的体内药代动力学数据。GI消化FI和F-II期间和之后,在水相中回收的A1260的量与C max和AUC 0-48h有关每种制剂的血浆浓度-时间分布曲线,并在体外-体内(IVIV)关系中产生了等级顺序。相比之下,将肠道消化前(t = 0分钟)和消化15分钟后在水相中回收的A1260的量与血浆浓度-时间曲线相关时,可获得类似的IVIV等级排序。但是,在肠道消化60分钟后,LbDDS在一步一步体外消化模型中的表现均与在两步消化模型中观察到的相反,并且在体内。与肠道消化模型相比,由于GI消化模型在两种LbDDS的LbDDS等级排序方面产生了更清晰的区别,因此发现它是研究和评估体内LbDDS行为的有希望的体外模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号