Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneous Removal of SO2 and Hg0 by Composite Oxidant NaClO/NaClO2 in a Packed Tower.
ACS Omega ( IF 4.1 ) Pub Date : 2020-07-14 , DOI: 10.1021/acsomega.0c00884 Hao Huang 1 , Hui Hu 1 , Maohong Fan 2 , Changchao Ruan 1 , Kunpeng Li 1 , Fan Zeng 3 , Liya Huang 1
ACS Omega ( IF 4.1 ) Pub Date : 2020-07-14 , DOI: 10.1021/acsomega.0c00884 Hao Huang 1 , Hui Hu 1 , Maohong Fan 2 , Changchao Ruan 1 , Kunpeng Li 1 , Fan Zeng 3 , Liya Huang 1
Affiliation
|
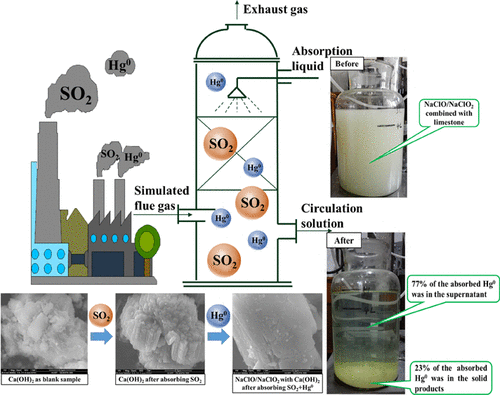
Based on the implementation of the global Minamata Convention, developing an efficient and economical technology for mercury reduction in coal-fired flue gas becomes a hotspot in the field of air pollution control. The composite oxidant NaClO/NaClO2 combined with limestone was used in the simultaneous removal of SO2 and Hg0 in this study, and the three-factor and four-level orthogonal experiments were performed in a packed tower. The influential sequence of various factors on SO2 and Hg0 removals was investigated through range analysis of the orthogonal experiments. Results showed that factors affecting desulfurization was C > A > B (liquid–gas ratio > oxidant concentration ratio > initial pH of absorption liquid), while factors affecting Hg0 removal was A > C > B (oxidant concentration ratio > liquid–gas ratio > initial pH of absorption liquid). Optimum conditions of simultaneous desulfurization and demercuration by NaClO/NaClO2 were A4B1C4; that is, the oxidant concentration ratio was 10/4 (mmol/L:mmol/L), the initial pH was 5, and the liquid–gas ratio was 18 (L/m3). The simultaneous removal efficiencies of SO2 and Hg0 reached 99.5 and 85.4% under these optimum conditions, respectively. Analysis of the characteristics of the solid products showed that the main products of the wet oxidation were CaSO4 and CaSO3. Analysis of the existing form of oxidized mercury showed that 23% of mercury was in the gypsum, while 77% was in the supernatant. Results of this research would provide a practical reference for promoting the simultaneous removal of SO2 and Hg0 by NaClO/NaClO2 with limestone in industrial application.
中文翻译:

在填充塔中通过复合氧化剂NaClO / NaClO2同时去除SO2和Hg0。
在执行全球水Min公约的基础上,开发一种高效经济的技术来减少燃煤烟气中的汞成为空气污染控制领域的热点。本研究采用复合氧化剂NaClO / NaClO 2结合石灰石同时去除SO 2和Hg 0,并在填料塔中进行了三因素和四能级正交试验。各种因素对SO 2和Hg 0的影响顺序通过正交实验的范围分析研究了去除率。结果表明,影响脱硫的因素为C> A> B(液气比>氧化剂浓度比>吸收液的初始pH),而影响Hg 0去除的因素为A> C> B(氧化剂浓度比>液气比) >吸收液的初始pH)。NaClO / NaClO 2同时脱硫和脱汞的最佳条件是A4B1C4。也就是说,氧化剂的浓度比为10/4(mmol / L:mmol / L),初始pH为5,液气比为18(L / m 3)。同时去除SO 2和Hg 0的效率在这些最佳条件下分别达到99.5%和85.4%。对固体产物的特征分析表明,湿式氧化的主要产物为CaSO 4和CaSO 3。对现有形式的氧化汞的分析表明,石膏中23%的汞,而上清液中77%的汞。该研究结果为促进石灰石中NaClO / NaClO 2同时脱除SO 2和Hg 0提供了实用参考。
更新日期:2020-07-28
中文翻译:

在填充塔中通过复合氧化剂NaClO / NaClO2同时去除SO2和Hg0。
在执行全球水Min公约的基础上,开发一种高效经济的技术来减少燃煤烟气中的汞成为空气污染控制领域的热点。本研究采用复合氧化剂NaClO / NaClO 2结合石灰石同时去除SO 2和Hg 0,并在填料塔中进行了三因素和四能级正交试验。各种因素对SO 2和Hg 0的影响顺序通过正交实验的范围分析研究了去除率。结果表明,影响脱硫的因素为C> A> B(液气比>氧化剂浓度比>吸收液的初始pH),而影响Hg 0去除的因素为A> C> B(氧化剂浓度比>液气比) >吸收液的初始pH)。NaClO / NaClO 2同时脱硫和脱汞的最佳条件是A4B1C4。也就是说,氧化剂的浓度比为10/4(mmol / L:mmol / L),初始pH为5,液气比为18(L / m 3)。同时去除SO 2和Hg 0的效率在这些最佳条件下分别达到99.5%和85.4%。对固体产物的特征分析表明,湿式氧化的主要产物为CaSO 4和CaSO 3。对现有形式的氧化汞的分析表明,石膏中23%的汞,而上清液中77%的汞。该研究结果为促进石灰石中NaClO / NaClO 2同时脱除SO 2和Hg 0提供了实用参考。



























 京公网安备 11010802027423号
京公网安备 11010802027423号