当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor Necrosis Factor-α Trimer Disassembly and Inactivation via Peptide-Small Molecule Synergy.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-07-14 , DOI: 10.1021/acschembio.0c00313 James W Checco 1 , Geoffrey A Eddinger 1 , Nicholas J Rettko 1 , Alexander R Chartier 1 , Samuel H Gellman 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-07-14 , DOI: 10.1021/acschembio.0c00313 James W Checco 1 , Geoffrey A Eddinger 1 , Nicholas J Rettko 1 , Alexander R Chartier 1 , Samuel H Gellman 1
Affiliation
|
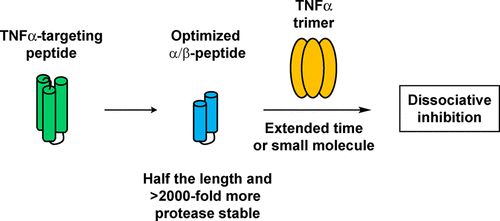
Aberrant signaling by tumor necrosis factor-α (TNFα) is associated with inflammatory diseases that can be treated with engineered proteins that inhibit binding of this cytokine to cell-surface receptors. Despite these clinical successes, there is considerable interest in the development of smaller antagonists of TNFα-receptor interactions. We describe a new 29-residue α/β-peptide, a molecule that contains three β-amino acid residues and three α-aminoisobutryic acid (Aib) residues, that displays potent inhibition of TNFα binding to TNFα receptor 1 (TNFR1) and rescues cells from TNFα-induced death. The complement of nonproteinogenic residues renders this α/β-peptide highly resistant to proteolysis, relative to all-α analogues. The mechanism of inhibitory action of the new 29-mer involves disruption of the trimeric TNFα quaternary structure, which prevents productive binding to TNFα receptors. Unexpectedly, we discovered that peptide-induced trimer disruption can be promoted by structurally diverse small molecules, including a detergent commonly used during selection procedures. The discovery of this synergistic effect provides a new context for understanding previous reports on peptidic antagonists of TNFα-receptor interactions and suggests new avenues for future efforts to block signaling via proteins with an active form that is oligomeric, including other members of the TNFα family.
中文翻译:

肿瘤坏死因子-α 三聚体分解和通过肽-小分子协同失活。
肿瘤坏死因子-α (TNFα) 的异常信号传导与炎症性疾病有关,可以用工程蛋白治疗,抑制这种细胞因子与细胞表面受体的结合。尽管取得了这些临床成功,但人们对开发更小的 TNFα-受体相互作用拮抗剂仍有相当大的兴趣。我们描述了一种新的 29 个残基 α/β-肽,一种包含三个 β-氨基酸残基和三个 α-氨基异丁酸 (Aib) 残基的分子,它显示出对 TNFα 与 TNFα 受体 1 (TNFR1) 结合的有效抑制并拯救TNFα 诱导的死亡细胞。相对于全 α 类似物,非蛋白质残基的补充使这种 α/β-肽对蛋白水解具有高度抗性。新的 29 聚体的抑制作用机制涉及破坏三聚体 TNFα 四级结构,这会阻止与 TNFα 受体的有效结合。出乎意料的是,我们发现结构不同的小分子可以促进肽诱导的三聚体破坏,包括在选择过程中常用的去污剂。这种协同效应的发现为理解先前关于 TNFα-受体相互作用的肽拮抗剂的报道提供了新的背景,并为未来通过具有寡聚活性形式的蛋白质(包括 TNFα 家族的其他成员)阻断信号传导的努力提出了新的途径。
更新日期:2020-08-21
中文翻译:

肿瘤坏死因子-α 三聚体分解和通过肽-小分子协同失活。
肿瘤坏死因子-α (TNFα) 的异常信号传导与炎症性疾病有关,可以用工程蛋白治疗,抑制这种细胞因子与细胞表面受体的结合。尽管取得了这些临床成功,但人们对开发更小的 TNFα-受体相互作用拮抗剂仍有相当大的兴趣。我们描述了一种新的 29 个残基 α/β-肽,一种包含三个 β-氨基酸残基和三个 α-氨基异丁酸 (Aib) 残基的分子,它显示出对 TNFα 与 TNFα 受体 1 (TNFR1) 结合的有效抑制并拯救TNFα 诱导的死亡细胞。相对于全 α 类似物,非蛋白质残基的补充使这种 α/β-肽对蛋白水解具有高度抗性。新的 29 聚体的抑制作用机制涉及破坏三聚体 TNFα 四级结构,这会阻止与 TNFα 受体的有效结合。出乎意料的是,我们发现结构不同的小分子可以促进肽诱导的三聚体破坏,包括在选择过程中常用的去污剂。这种协同效应的发现为理解先前关于 TNFα-受体相互作用的肽拮抗剂的报道提供了新的背景,并为未来通过具有寡聚活性形式的蛋白质(包括 TNFα 家族的其他成员)阻断信号传导的努力提出了新的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号