当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low Temperature Activation of Methane on Metal-Oxides and Complex Interfaces: Insights from Surface Science.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.accounts.0c00194 Sanjaya D Senanayake 1 , José A Rodriguez 1 , Jason F Weaver 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.accounts.0c00194 Sanjaya D Senanayake 1 , José A Rodriguez 1 , Jason F Weaver 2
Affiliation
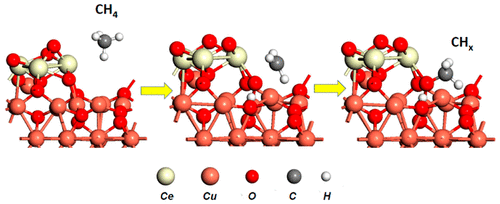
|
The abundance of cheap, natural gas has transformed the energy landscape, whereby revealing new possibilities for sustainable chemical technologies or impacting those that have relied on traditional fossil fuels. The primary component, methane, is underutilized and wastefully exhausted, leading to anthropogenic global warming. Historically, the manipulation of methane remained “clavis aurea,” an insurmountable yet rewarding challenge and thus the focus of intense research. This is primarily due to an inability to dissociate C–H bonds in methane selectively, which requires a high energy penalty and is an essential prerequisite for the direct conversion of methane into a large set of value-added products. The discovery of such processes would promise an energy gainful use of natural gas benefiting several essential chemical processes associated with C1 chemistry. This first C–H bond dissociation step of the methane molecule appears in numerous catalytic mechanisms as the rate-determining step or most essential barrier sequence for all subsequent steps that follow in the production of C–C, C–O, or Cx–Hy–Oz bonds found in value added products. A main goal is to catalytically reduce the energy barrier for the first C–H bond dissociation to be able to achieve the activation of methane at low or moderate temperatures. As such there is great value in understanding the fundamental nature of the active sites responsible for bond breaking or formation and thus be able to facilitate better control of this chemistry, leading to the development of new technologies for fuel production and chemical conversion. Surface science studies offer enhanced perspectives for a careful manipulation of bonds over the last layer atoms of catalyst surfaces, an essential factor for the design of atomically precise catalysts and unravelling of the reaction mechanism. With the advent of new surface imaging, spectroscopy, and in situ tools, it has been possible to decipher the surface chemistry of complex materials systems and further our understanding of atomic active sites on the surfaces of metals, oxides, and carbides or metal–oxide and metal–carbide interfaces. The once considered near impossible step of C–H bond activation is now observed at low temperatures with high propensity over a collection of oxide, metal–oxide, and metal–carbide systems in a conventional or inverse configuration (oxide or carbide on metal). The enabling of C–H activation at low temperature has opened interesting possibilities for the specific production of chemicals such as methanol directly from methane, a step toward facile synthesis of liquid fuels. We highlight the most recent of these results and present the key aspects of active site configurations engineered from surface science studies which enable such a simple reactive event through careful manipulation of the last surface layer of atoms found in the catalyst structure. New concepts which help in the activation and conversion of methane are discussed.
中文翻译:

金属氧化物和复杂界面上甲烷的低温活化:表面科学的见解。
大量廉价的天然气已经改变了能源格局,从而揭示了可持续化学技术的新可能性或对那些依赖传统化石燃料的技术产生了影响。甲烷的主要成分未得到充分利用和浪费,导致人为的全球变暖。从历史上看,甲烷的处理仍然是“ clavis aurea””,这是一项无法克服但又有意义的挑战,因此也是深入研究的重点。这主要是由于无法选择性地分解甲烷中的C–H键,这需要很高的能量消耗,并且是将甲烷直接转化为大量增值产品的必要前提。这样的过程的发现将有望使天然气的能量利用效率受益,这将有益于与C1化学反应相关的几种基本化学过程。甲烷分子的第一个C–H键解离步骤出现在众多催化机理中,作为速率决定步骤或最重要的屏障顺序,是后续生产C–C,C–O或C x –的所有后续步骤H y –O z增值产品中发现的债券。一个主要目标是催化降低首次C–H键解离的能垒,从而能够在低温或中温下实现甲烷的活化。因此,了解负责键断裂或形成的活性位点的基本性质具有极大的价值,因此能够促进更好地控制这种化学反应,从而导致了用于燃料生产和化学转化的新技术的发展。表面科学研究为仔细操纵催化剂表面最后一层原子上的键提供了增强的视角,这是设计原子精确催化剂和阐明反应机理的重要因素。随着新的表面成像,光谱学和原位技术的出现工具,有可能破译复杂材料系统的表面化学,并进一步加深我们对金属,氧化物和碳化物或金属-氧化物和金属-碳化物界面的原子活性位点的理解。现在曾经观察到曾经被认为几乎不可能实现的C–H键活化步骤,现在在低温下以常规或相反配置(金属上的氧化物或碳化物)在一系列氧化物,金属-氧化物和金属-碳酸盐体系上具有很高的倾向。在低温下启用C–H活化为直接从甲烷中直接生产特定的化学品(例如甲醇)开辟了有趣的可能性,这是向液态燃料轻松合成迈出的一步。我们重点介绍了这些结果中的最新成果,并介绍了由表面科学研究所设计的活性位点配置的关键方面,这些细节通过仔细操纵催化剂结构中发现的原子的最后表面层而实现了这种简单的反应性事件。讨论了有助于甲烷活化和转化的新概念。
更新日期:2020-08-18
中文翻译:

金属氧化物和复杂界面上甲烷的低温活化:表面科学的见解。
大量廉价的天然气已经改变了能源格局,从而揭示了可持续化学技术的新可能性或对那些依赖传统化石燃料的技术产生了影响。甲烷的主要成分未得到充分利用和浪费,导致人为的全球变暖。从历史上看,甲烷的处理仍然是“ clavis aurea””,这是一项无法克服但又有意义的挑战,因此也是深入研究的重点。这主要是由于无法选择性地分解甲烷中的C–H键,这需要很高的能量消耗,并且是将甲烷直接转化为大量增值产品的必要前提。这样的过程的发现将有望使天然气的能量利用效率受益,这将有益于与C1化学反应相关的几种基本化学过程。甲烷分子的第一个C–H键解离步骤出现在众多催化机理中,作为速率决定步骤或最重要的屏障顺序,是后续生产C–C,C–O或C x –的所有后续步骤H y –O z增值产品中发现的债券。一个主要目标是催化降低首次C–H键解离的能垒,从而能够在低温或中温下实现甲烷的活化。因此,了解负责键断裂或形成的活性位点的基本性质具有极大的价值,因此能够促进更好地控制这种化学反应,从而导致了用于燃料生产和化学转化的新技术的发展。表面科学研究为仔细操纵催化剂表面最后一层原子上的键提供了增强的视角,这是设计原子精确催化剂和阐明反应机理的重要因素。随着新的表面成像,光谱学和原位技术的出现工具,有可能破译复杂材料系统的表面化学,并进一步加深我们对金属,氧化物和碳化物或金属-氧化物和金属-碳化物界面的原子活性位点的理解。现在曾经观察到曾经被认为几乎不可能实现的C–H键活化步骤,现在在低温下以常规或相反配置(金属上的氧化物或碳化物)在一系列氧化物,金属-氧化物和金属-碳酸盐体系上具有很高的倾向。在低温下启用C–H活化为直接从甲烷中直接生产特定的化学品(例如甲醇)开辟了有趣的可能性,这是向液态燃料轻松合成迈出的一步。我们重点介绍了这些结果中的最新成果,并介绍了由表面科学研究所设计的活性位点配置的关键方面,这些细节通过仔细操纵催化剂结构中发现的原子的最后表面层而实现了这种简单的反应性事件。讨论了有助于甲烷活化和转化的新概念。











































 京公网安备 11010802027423号
京公网安备 11010802027423号