当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and evaluation of novel 1,2,4‐triazolo‐[3,4‐b]‐1,3,4‐thiadiazole tethered chalcone hybrids as potential anticancer agents
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-13 , DOI: 10.1002/jhet.4047 Sushmitha Bujji 1 , Praveen Kumar Edigi 2 , Naikal James Prameela Subhashini 1, 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-13 , DOI: 10.1002/jhet.4047 Sushmitha Bujji 1 , Praveen Kumar Edigi 2 , Naikal James Prameela Subhashini 1, 2
Affiliation
|
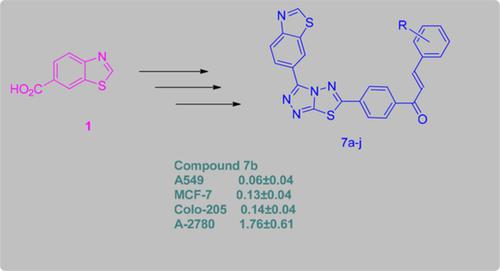
A series of novel 1,2,4‐triazolo‐[3,4‐b]‐1,3,4‐thiadiazole tethered chalcone derivatives were designed synthesized by using conventional as well as microwave assisted methods and characterized by 1H NMR, 13C NMR and mass spectral analysis. Microwave assisted synthesis caused an improvement in yield and significant reduction in the reaction time of all the derivatives. All the new synthesized derivatives were further evaluated for their anti‐cancer activity against selected four human cancer cell lines; A549 (Lung), MCF‐7 (Breast), Colo‐205 (Colon) and A2780 (Ovarian). Among the synthesized compounds, 7b, 7c, 7d, 7e, 7g and 7h exhibited potent anticancer activity compared to control drug (etoposide).
中文翻译:

新型1,2,4-三唑并[3,4-b] -1,3,4-噻二唑系链查尔酮杂化物的合成和评价
设计了一系列新颖的1,2,4-三唑并[3,4-b] -1,3,4-噻二唑系链查尔酮衍生物,使用常规方法和微波辅助方法合成并通过1 H NMR表征,13 13 C NMR和质谱分析。微波辅助合成导致所有衍生物的产率提高和反应时间的显着减少。进一步评估了所有新合成的衍生物对选定的四种人类癌细胞系的抗癌活性;A549(肺),MCF-7(乳腺癌),Colo-205(结肠)和A2780(卵巢)。在合成的化合物中,7b,7c,7d,7e,7g和7h 与对照药物(依托泊苷)相比,具有较强的抗癌活性。
更新日期:2020-09-08
中文翻译:

新型1,2,4-三唑并[3,4-b] -1,3,4-噻二唑系链查尔酮杂化物的合成和评价
设计了一系列新颖的1,2,4-三唑并[3,4-b] -1,3,4-噻二唑系链查尔酮衍生物,使用常规方法和微波辅助方法合成并通过1 H NMR表征,13 13 C NMR和质谱分析。微波辅助合成导致所有衍生物的产率提高和反应时间的显着减少。进一步评估了所有新合成的衍生物对选定的四种人类癌细胞系的抗癌活性;A549(肺),MCF-7(乳腺癌),Colo-205(结肠)和A2780(卵巢)。在合成的化合物中,7b,7c,7d,7e,7g和7h 与对照药物(依托泊苷)相比,具有较强的抗癌活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号