当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vitro Reconstitution Reveals a Central Role for the N-Oxygenase PvfB in (Dihydro)pyrazine-N-oxide and Valdiazen Biosynthesis.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-14 , DOI: 10.1002/anie.202005554 Gina L Morgan 1 , Bo Li 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-14 , DOI: 10.1002/anie.202005554 Gina L Morgan 1 , Bo Li 1
Affiliation
|
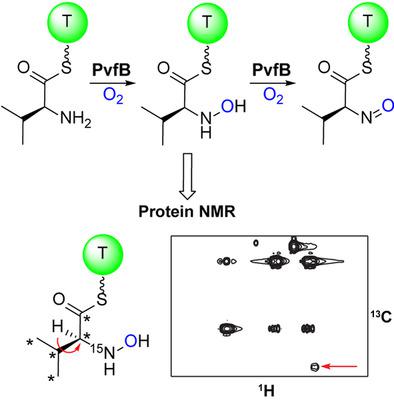
The Pseudomonas virulence factor (pvf) operon is essential for the biosynthesis of two very different natural product scaffolds: the (dihydro)pyrazine‐N‐oxides and the diazeniumdiolate, valdiazen. PvfB is a member of the non‐heme diiron N‐oxygenase enzyme family that commonly convert anilines to their nitroaromatic counterparts. In contrast, we show that PvfB catalyzes N‐oxygenation of the α‐amine of valine, first to the hydroxylamine and then the nitroso, while linked to the carrier protein of PvfC. PvfB modification of PvfC‐tethered valine was observed directly by protein NMR spectroscopy, establishing the intermediacy of the hydroxylamine. This work reveals a central role for PvfB in the biosynthesis of (dihydro)pyrazine‐N‐oxides and valdiazen.
中文翻译:

体外重组揭示了 N-氧化酶 PvfB 在(二氢)吡嗪-N-氧化物和 Valdiazen 生物合成中的核心作用。
假单胞菌毒力因子 (pvf) 操纵子对于两种截然不同的天然产物支架的生物合成必不可少:(二氢)吡嗪-N-氧化物和二氮烯二烯鎓盐酸盐,valdiazen。PvfB 是非血红素二铁 N-加氧酶家族的成员,通常将苯胺转化为其硝基芳族化合物。相比之下,我们表明 PvfB 催化缬氨酸的 α-胺的 N-氧化,首先是羟胺,然后是亚硝基,同时与 PvfC 的载体蛋白相连。通过蛋白质核磁共振光谱直接观察到 PvfC 连接的缬氨酸的 PvfB 修饰,确定了羟胺的中间体。这项工作揭示了 PvfB 在(二氢)吡嗪-N-氧化物和伐地嗪的生物合成中的核心作用。
更新日期:2020-07-14
中文翻译:

体外重组揭示了 N-氧化酶 PvfB 在(二氢)吡嗪-N-氧化物和 Valdiazen 生物合成中的核心作用。
假单胞菌毒力因子 (pvf) 操纵子对于两种截然不同的天然产物支架的生物合成必不可少:(二氢)吡嗪-N-氧化物和二氮烯二烯鎓盐酸盐,valdiazen。PvfB 是非血红素二铁 N-加氧酶家族的成员,通常将苯胺转化为其硝基芳族化合物。相比之下,我们表明 PvfB 催化缬氨酸的 α-胺的 N-氧化,首先是羟胺,然后是亚硝基,同时与 PvfC 的载体蛋白相连。通过蛋白质核磁共振光谱直接观察到 PvfC 连接的缬氨酸的 PvfB 修饰,确定了羟胺的中间体。这项工作揭示了 PvfB 在(二氢)吡嗪-N-氧化物和伐地嗪的生物合成中的核心作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号