当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An integrative toolbox to unlock the structure and dynamics of protein–surfactant complexes
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0na00194e Adrian Sanchez-Fernandez 1 , Carl Diehl 2 , Judith E Houston 3 , Anna E Leung 3 , James P Tellam 4 , Sarah E Rogers 4 , Sylvain Prevost 5 , Stefan Ulvenlund 1, 6 , Helen Sjögren 7 , Marie Wahlgren 1, 6
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0na00194e Adrian Sanchez-Fernandez 1 , Carl Diehl 2 , Judith E Houston 3 , Anna E Leung 3 , James P Tellam 4 , Sarah E Rogers 4 , Sylvain Prevost 5 , Stefan Ulvenlund 1, 6 , Helen Sjögren 7 , Marie Wahlgren 1, 6
Affiliation
|
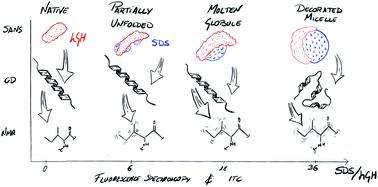
The interactions between protein and surfactants play an important role in the stability and performance of formulated products. Due to the high complexity of such interactions, multi-technique approaches are required to study these systems. Here, an integrative approach is used to investigate the various interactions in a model system composed of human growth hormone and sodium dodecyl sulfate. Contrast variation small-angle neutron scattering was used to obtain information on the structure of the protein, surfactant aggregates and surfactant–protein complexes. 1H and 1H–13C HSQC nuclear magnetic resonance spectroscopy was employed to probe the local structure and dynamics of specific amino acids upon surfactant addition. Through the combination of these advanced methods with fluorescence spectroscopy, circular dichroism and isothermal titration calorimetry, it was possible to identify the interaction mechanisms between the surfactant and the protein in the pre- and post-micellar regimes, and interconnect the results from different techniques. As such, the protein was revealed to evolve from a partially unfolded conformation at low SDS concentration to a molten globule at intermediate concentrations, where the protein conformation and local dynamics of hydrophobic amino acids are partially affected compared to the native state. At higher surfactant concentrations the local structure of the protein appears disrupted, and a decorated micelle structure is observed, where the protein is wrapped around a surfactant assembly. Importantly, this integrative approach allows for the identification of the characteristic fingerprints of complex transitions as seen by each technique, and establishes a methodology for an in-detail study of surfactant–protein systems.
中文翻译:

解锁蛋白质-表面活性剂复合物结构和动力学的综合工具箱
蛋白质和表面活性剂之间的相互作用对于配制产品的稳定性和性能起着重要作用。由于这种相互作用的高度复杂性,需要多种技术方法来研究这些系统。在这里,采用综合方法研究由人生长激素和十二烷基硫酸钠组成的模型系统中的各种相互作用。使用对比度变化小角中子散射来获取蛋白质、表面活性剂聚集体和表面活性剂-蛋白质复合物的结构信息。采用1 H 和1 H– 13 C HSQC 核磁共振波谱来探测添加表面活性剂后特定氨基酸的局部结构和动力学。通过将这些先进方法与荧光光谱、圆二色性和等温滴定量热法相结合,可以确定胶束前和胶束后表面活性剂与蛋白质之间的相互作用机制,并将不同技术的结果互连起来。因此,蛋白质从低 SDS 浓度下的部分展开构象演化为中等浓度的熔球,其中与天然状态相比,蛋白质构象和疏水性氨基酸的局部动力学受到部分影响。在较高的表面活性剂浓度下,蛋白质的局部结构似乎被破坏,并且观察到修饰的胶束结构,其中蛋白质包裹在表面活性剂组装体周围。 重要的是,这种综合方法可以识别每种技术所看到的复杂转变的特征指纹,并建立了表面活性剂-蛋白质系统的详细研究方法。
更新日期:2020-09-16
中文翻译:

解锁蛋白质-表面活性剂复合物结构和动力学的综合工具箱
蛋白质和表面活性剂之间的相互作用对于配制产品的稳定性和性能起着重要作用。由于这种相互作用的高度复杂性,需要多种技术方法来研究这些系统。在这里,采用综合方法研究由人生长激素和十二烷基硫酸钠组成的模型系统中的各种相互作用。使用对比度变化小角中子散射来获取蛋白质、表面活性剂聚集体和表面活性剂-蛋白质复合物的结构信息。采用1 H 和1 H– 13 C HSQC 核磁共振波谱来探测添加表面活性剂后特定氨基酸的局部结构和动力学。通过将这些先进方法与荧光光谱、圆二色性和等温滴定量热法相结合,可以确定胶束前和胶束后表面活性剂与蛋白质之间的相互作用机制,并将不同技术的结果互连起来。因此,蛋白质从低 SDS 浓度下的部分展开构象演化为中等浓度的熔球,其中与天然状态相比,蛋白质构象和疏水性氨基酸的局部动力学受到部分影响。在较高的表面活性剂浓度下,蛋白质的局部结构似乎被破坏,并且观察到修饰的胶束结构,其中蛋白质包裹在表面活性剂组装体周围。 重要的是,这种综合方法可以识别每种技术所看到的复杂转变的特征指纹,并建立了表面活性剂-蛋白质系统的详细研究方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号