当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Placement of tyrosine residues as a design element for tuning the phase transition of elastin-peptide-containing conjugates: experiments and simulations
Molecular Systems Design & Engineering ( IF 3.6 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0me00051e Phillip A Taylor 1 , Haofu Huang 2 , Kristi L Kiick 2 , Arthi Jayaraman 1
Molecular Systems Design & Engineering ( IF 3.6 ) Pub Date : 2020-07-13 , DOI: 10.1039/d0me00051e Phillip A Taylor 1 , Haofu Huang 2 , Kristi L Kiick 2 , Arthi Jayaraman 1
Affiliation
|
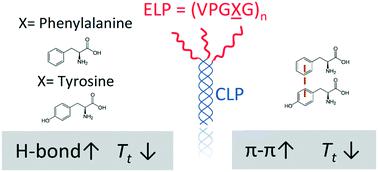
Elastin-like polypeptides (ELP) have been widely used in the biomaterials community due to their controllable, thermoresponsive properties and biocompatibility. Motivated by our previous work on the effect of tryptophan (W) substitutions on the LCST-like transitions of short ELPs, we studied a series of short ELPs containing tyrosine (Y) and/or phenylalanine (F) guest residues with only 5 or 6 pentapeptide repeat units. A combination of experiments and molecular dynamics (MD) simulations illustrated that the substitution of F with Y guest residues impacted the transition temperature (Tt) of short ELPs when conjugated to collagen-like-peptides (CLP), with a reduction in the transition temperature observed only after substitution of at least two residues. Placement of the Y residues near the N-terminal end of the ELP, away from the tethering point to the CLP, resulted in a lower Tt than that observed for peptides with the Y residues near the tethering point. Atomistic and coarse-grained MD simulations indicated an increase in intra- and inter-peptide hydrogen bonds in systems containing Y guest residues that are suggested to enhance the ability of the peptides to coacervate, with a concomitantly lower Tt. Simulations also revealed that the placement of Y-containing pentads near the N-terminus (i.e., away from the CLP tethering point) versus C-terminus of the ELP led to more π–π stacking interactions at low temperatures, in agreement with our experimental observations of a lower Tt. Overall, this study provides mechanistic insights into the driving forces for the LCST-like transitions of ELPs and offers additional means for tuning the Tt of short ELPs for biomedical applications such as on-demand drug delivery and tissue engineering.
中文翻译:

酪氨酸残基的放置作为调整含有弹性蛋白肽的缀合物的相变的设计元素:实验和模拟
类弹性蛋白多肽(ELP)由于其可控、热响应特性和生物相容性而在生物材料领域得到广泛应用。受我们之前关于色氨酸 (W) 取代对短 ELP 的 LCST 样转变影响的研究的启发,我们研究了一系列仅含有 5 或 6 个酪氨酸 (Y) 和/或苯丙氨酸 (F) 客体残基的短 ELP五肽重复单元。实验和分子动力学 (MD) 模拟相结合表明,当与类胶原肽 (CLP) 缀合时,用 Y 客体残基取代 F 会影响短 ELP 的转变温度 ( T t ),从而降低转变温度仅在至少两个残基取代后观察到的温度。将 Y 残基放置在 ELP N 末端附近,远离 CLP 的束缚点,导致 T t低于 Y 残基靠近束缚点的肽所观察到的T t 。原子和粗粒度 MD 模拟表明,含有 Y 客体残基的系统中肽内和肽间氢键增加,这被认为增强了肽凝聚的能力,同时 T t较低。模拟还表明,将含 Y 的五元组放置在 ELP 的 N 末端附近(即远离 CLP 束缚点)与ELP 的 C 末端相比,会导致低温下更多的 π-π 堆积相互作用,这与我们的实验一致。观察到较低的T t。总体而言,这项研究提供了对 ELP 类似 LCST 转变的驱动力的机制见解,并提供了调整短 ELP 的T t的额外方法,以用于生物医学应用,例如按需药物输送和组织工程。
更新日期:2020-08-17
中文翻译:

酪氨酸残基的放置作为调整含有弹性蛋白肽的缀合物的相变的设计元素:实验和模拟
类弹性蛋白多肽(ELP)由于其可控、热响应特性和生物相容性而在生物材料领域得到广泛应用。受我们之前关于色氨酸 (W) 取代对短 ELP 的 LCST 样转变影响的研究的启发,我们研究了一系列仅含有 5 或 6 个酪氨酸 (Y) 和/或苯丙氨酸 (F) 客体残基的短 ELP五肽重复单元。实验和分子动力学 (MD) 模拟相结合表明,当与类胶原肽 (CLP) 缀合时,用 Y 客体残基取代 F 会影响短 ELP 的转变温度 ( T t ),从而降低转变温度仅在至少两个残基取代后观察到的温度。将 Y 残基放置在 ELP N 末端附近,远离 CLP 的束缚点,导致 T t低于 Y 残基靠近束缚点的肽所观察到的T t 。原子和粗粒度 MD 模拟表明,含有 Y 客体残基的系统中肽内和肽间氢键增加,这被认为增强了肽凝聚的能力,同时 T t较低。模拟还表明,将含 Y 的五元组放置在 ELP 的 N 末端附近(即远离 CLP 束缚点)与ELP 的 C 末端相比,会导致低温下更多的 π-π 堆积相互作用,这与我们的实验一致。观察到较低的T t。总体而言,这项研究提供了对 ELP 类似 LCST 转变的驱动力的机制见解,并提供了调整短 ELP 的T t的额外方法,以用于生物医学应用,例如按需药物输送和组织工程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号