当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Fabrication of Nanoparticles with Dual-Targeting Ligands for Precise Hepatocellular Carcinoma Therapy In Vitro and In Vivo.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.molpharmaceut.0c00327 Ya Xiang 1 , Wen Huang 1 , Cong Huang 1 , Jinrong Long 1 , Yangchun Zhou 1 , Yufeng Liu 1 , Siyue Tang 1 , Dong-Xiu He 1 , Xiang-Wen Tan 1 , Hua Wei 1 , Cui-Yun Yu 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.molpharmaceut.0c00327 Ya Xiang 1 , Wen Huang 1 , Cong Huang 1 , Jinrong Long 1 , Yangchun Zhou 1 , Yufeng Liu 1 , Siyue Tang 1 , Dong-Xiu He 1 , Xiang-Wen Tan 1 , Hua Wei 1 , Cui-Yun Yu 1
Affiliation
|
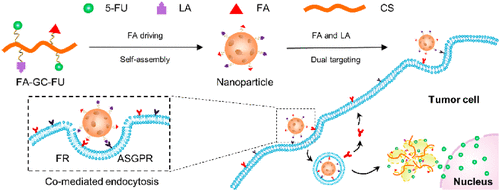
Efficient hepatocellular carcinoma (HCC) therapy remains a significant challenge due to the unsatisfactory targeting efficiency of nanoparticles (NPs) with either a passive targeting or a single active targeting property. Although a dual-targeting mechanism-based strategy can promote the partial targeting efficiency, most of the reported NPs with dual-targeting properties generally suffer from sophisticated chemical design, multistep synthesis, and purification procedures, leading to batch-to-batch variation and difficulties in scalable production. To develop a facile yet efficient strategy toward dual-targeting ligand-functionalized NPs for precise HCC therapy and potential clinical translation, folic acid (FA) was readily introduced as a hydrophobic and targeting component to a hydrophilic macromolecular prodrug, galactosylated chitosan-5-fluorouracil acetic acid (GC-FU), to afford FA-GC-FU formulation that can self-assemble into NPs driven by the solubility variation of FA and GC-FU without the necessity of previously used physical cross-linking. The resulting nanoparticles of FA-GC-FU can target the overexpressed asialoglycoprotein receptors (ASGPRs) and folate receptors (FRs) on the surface of HCC cells, respectively, via the FA and lactobionic acid (LA) residues exposed on the surface of the NPs, leading to the maximized targeting efficiency of HCC and minimized nonspecific uptake by normal hepatocytes in vitro and in vivo. Therefore, this study not only developed a simple yet efficient strategy toward a facile fabrication of NPs with dual-targeting ligands but also presented a precise therapeutic platform for HCC with great potential for clinical translation.
中文翻译:

带有双重目标配体的纳米粒子的简便制备,可用于体外和体内精确肝细胞癌治疗。
由于具有被动靶向或单个主动靶向特性的纳米颗粒(NP)的靶向效率不令人满意,有效的肝细胞癌(HCC)治疗仍然是一项重大挑战。尽管基于双重靶向机制的策略可以提高部分靶向效率,但大多数报道的具有双重靶向性质的NP通常都具有复杂的化学设计,多步合成和纯化程序,从而导致批次间的差异和困难。在可扩展的生产中。为了开发一种针对双重目标的配体官能化NPs的简便而有效的策略以进行精确的HCC治疗和潜在的临床翻译,叶酸(FA)作为疏水性和靶向性成分已很容易地引入亲水性大分子前药中,半乳糖基化壳聚糖-5-氟尿嘧啶乙酸(GC-FU),以提供FA-GC-FU制剂,该制剂可以由FA和GC-FU的溶解度变化驱动而自组装成NP,而无需先前使用的物理交联。所得的FA-GC-FU纳米颗粒可以分别通过暴露在NPs表面的FA和乳糖酸(LA)残基靶向HCC细胞表面过表达的去唾液酸糖蛋白受体(ASGPR)和叶酸受体(FRs)。 ,从而使HCC的靶向效率最大化,并且正常肝细胞的非特异性摄取最小化体外和体内。因此,这项研究不仅为简便地制备具有双重靶向配体的NP制定了简单而有效的策略,而且为HCC提供了精确的治疗平台,具有很大的临床翻译潜力。
更新日期:2020-09-09
中文翻译:

带有双重目标配体的纳米粒子的简便制备,可用于体外和体内精确肝细胞癌治疗。
由于具有被动靶向或单个主动靶向特性的纳米颗粒(NP)的靶向效率不令人满意,有效的肝细胞癌(HCC)治疗仍然是一项重大挑战。尽管基于双重靶向机制的策略可以提高部分靶向效率,但大多数报道的具有双重靶向性质的NP通常都具有复杂的化学设计,多步合成和纯化程序,从而导致批次间的差异和困难。在可扩展的生产中。为了开发一种针对双重目标的配体官能化NPs的简便而有效的策略以进行精确的HCC治疗和潜在的临床翻译,叶酸(FA)作为疏水性和靶向性成分已很容易地引入亲水性大分子前药中,半乳糖基化壳聚糖-5-氟尿嘧啶乙酸(GC-FU),以提供FA-GC-FU制剂,该制剂可以由FA和GC-FU的溶解度变化驱动而自组装成NP,而无需先前使用的物理交联。所得的FA-GC-FU纳米颗粒可以分别通过暴露在NPs表面的FA和乳糖酸(LA)残基靶向HCC细胞表面过表达的去唾液酸糖蛋白受体(ASGPR)和叶酸受体(FRs)。 ,从而使HCC的靶向效率最大化,并且正常肝细胞的非特异性摄取最小化体外和体内。因此,这项研究不仅为简便地制备具有双重靶向配体的NP制定了简单而有效的策略,而且为HCC提供了精确的治疗平台,具有很大的临床翻译潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号