当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogen/Oxygen Co-Doped Porous Carbon Derived from Biomass for Low-Pressure CO2 Capture
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.iecr.0c00006 Dawei Wu 1 , Jing Liu 1 , Yingju Yang 1 , Ying Zheng 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.iecr.0c00006 Dawei Wu 1 , Jing Liu 1 , Yingju Yang 1 , Ying Zheng 1
Affiliation
|
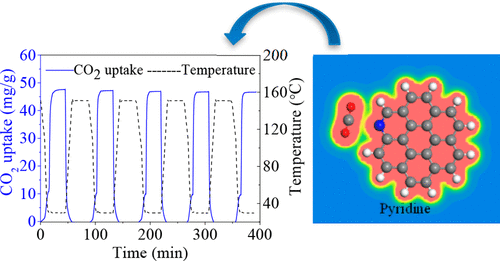
Nitrogen/oxygen co-doped porous carbon with excellent textural properties was synthesized from biomass for CO2 capture using the freeze-drying and nonthermal plasma treatment methods. Quantum chemical calculations were performed to reveal the CO2 adsorption mechanism. Various nitrogen and oxygen functional groups can be produced on the carbon surface during air plasma treatment. Plasma treatment hardly influences the morphology and textural properties of porous carbon. The plasma-modified sample PC-A10 exhibits the highest CO2 adsorption ability of 37.90 mg/g in the simulated flue gas at 30 °C. The superior capture performance is closely associated with the existence of nitrogen and oxygen functional groups produced from plasma treatment. The porous carbon shows excellent regeneration ability. CO2 adsorption capacity does not significantly degrade over five adsorption/desorption cycles. Kinetic analysis indicates that CO2 adsorption in porous carbon takes place at high rates. The pseudo-second-order and the Bangham adsorption models can describe well the CO2 adsorption kinetics. The results of DFT calculations indicate the physical sorption nature of CO2 adsorption by porous carbon. Pyridine-typed carbon surface has the highest attraction for CO2 molecules. No significant electrons are transferred between the CO2 molecule and the carbon surface.
中文翻译:

来自生物质的氮/氧共掺杂多孔碳,用于低压CO 2捕集
使用冷冻干燥和非热等离子体处理方法,从生物质中合成了具有优异质地特性的氮/氧共掺杂多孔碳,以捕获CO 2。进行量子化学计算以揭示CO 2吸附机理。在空气等离子体处理过程中,碳表面会产生各种氮和氧官能团。等离子体处理几乎不影响多孔碳的形态和质构特性。等离子体改性的样品PC-A10表现出最高的CO 2在30°C下模拟烟气中的吸附能力为37.90 mg / g。优异的捕获性能与等离子体处理产生的氮和氧官能团的存在密切相关。多孔碳显示出优异的再生能力。在五个吸附/解吸循环中,CO 2吸附能力不会明显降低。动力学分析表明,CO 2在多孔碳中的吸附速率很高。拟二级和Bangham吸附模型可以很好地描述CO 2吸附动力学。DFT计算的结果表明多孔碳吸附CO 2的物理吸附性质。吡啶型碳表面对CO的吸引力最高2个分子。没有明显的电子在CO 2分子和碳表面之间转移。
更新日期:2020-08-05
中文翻译:

来自生物质的氮/氧共掺杂多孔碳,用于低压CO 2捕集
使用冷冻干燥和非热等离子体处理方法,从生物质中合成了具有优异质地特性的氮/氧共掺杂多孔碳,以捕获CO 2。进行量子化学计算以揭示CO 2吸附机理。在空气等离子体处理过程中,碳表面会产生各种氮和氧官能团。等离子体处理几乎不影响多孔碳的形态和质构特性。等离子体改性的样品PC-A10表现出最高的CO 2在30°C下模拟烟气中的吸附能力为37.90 mg / g。优异的捕获性能与等离子体处理产生的氮和氧官能团的存在密切相关。多孔碳显示出优异的再生能力。在五个吸附/解吸循环中,CO 2吸附能力不会明显降低。动力学分析表明,CO 2在多孔碳中的吸附速率很高。拟二级和Bangham吸附模型可以很好地描述CO 2吸附动力学。DFT计算的结果表明多孔碳吸附CO 2的物理吸附性质。吡啶型碳表面对CO的吸引力最高2个分子。没有明显的电子在CO 2分子和碳表面之间转移。









































 京公网安备 11010802027423号
京公网安备 11010802027423号