Nature Catalysis ( IF 42.8 ) Pub Date : 2020-07-13 , DOI: 10.1038/s41929-020-0485-2 Hugo Helbert , Paco Visser , Johannes G. H. Hermens , Jeffrey Buter , Ben L. Feringa
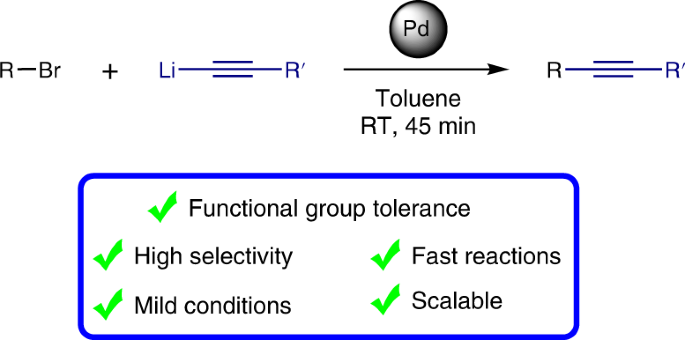
The incorporation of alkynes into organic molecules is one of the most valuable transformations for the formation of C–C bonds and provides a versatile handle for further modifications. The Sonogashira cross-coupling of acetylenes holds a prominent position among the suite of catalytic cross-coupling reactions that are key to modern synthesis. Here we present a method that is complementary to the Sonogashira reaction, demonstrating cross-coupling of lithium acetylides with aryl bromides. The reactions take place under ambient conditions with short reaction times, affording the corresponding aryl acetylenes in good to excellent yields while displaying a remarkable functional group tolerance for an organolithium reaction, allowing the presence of a variety of organolithium-sensitive carbonyl functionalities. This developed cross-coupling methodology offers ample opportunities to access a wide variety of acetylenes, as is illustrated by the facile preparation of key intermediates for chemical biology and optoelectronic materials.
中文翻译:

钯催化的乙炔锂交叉偶联
将炔烃掺入有机分子是形成C–C键的最有价值的转化之一,并为进一步修饰提供了一种通用的方法。乙炔的Sonogashira交叉偶联在一系列催化交叉偶联反应中占有重要地位,这是现代合成的关键。在这里,我们提出了一种与Sonogashira反应互补的方法,证明了乙炔锂与芳基溴的交叉偶联。反应在环境条件下以短的反应时间进行,以良好的产率至优异的产率提供相应的芳基乙炔,同时对有机锂反应显示出显着的官能团耐受性,从而允许存在多种有机锂敏感的羰基官能团。












































 京公网安备 11010802027423号
京公网安备 11010802027423号