当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amide-Modified RNA: Using Protein Backbone to Modulate Function of Short Interfering RNAs.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.accounts.0c00249 Venubabu Kotikam 1 , Eriks Rozners 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.accounts.0c00249 Venubabu Kotikam 1 , Eriks Rozners 1
Affiliation
|
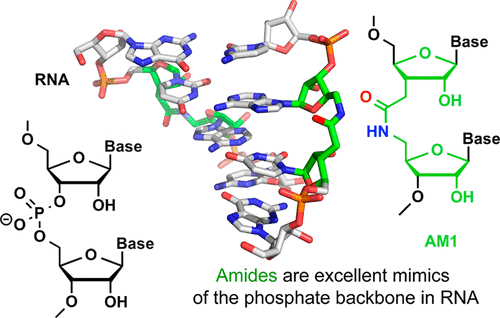
RNA-based technologies to control gene expression, such as RNA interference (RNAi) and CRISPR-Cas9, have become powerful tools in molecular biology and genomics. The exciting potential that RNAi and CRISPR-Cas9 may also become new therapeutic approaches has reinvigorated interest in chemically modifying RNA to improve its properties for in vivo applications. Chemical modifications can improve enzymatic stability, in vivo delivery, cellular uptake, and sequence specificity as well as minimize off-target activity of short interfering RNAs (siRNAs) and CRISPR associated RNAs. While numerous good solutions for improving stability toward enzymatic degradation have emerged, optimization of the latter functional properties remains challenging. In this Account, we discuss synthesis, structure, and biological activity of novel nonionic analogues of RNA that have the phosphodiester backbone replaced by amide linkages (AM1). Our long-term goal is to use the amide backbone to improve the stability and specificity of siRNAs and other functional RNAs. Our work in this area was motivated by early discoveries that nonionic backbone modifications, including AM1, did not disturb the overall structure or thermal stability of RNA duplexes. We hypothesized that the reduced negative charge and hydrophobic nature of the AM1 backbone modification might be useful in optimizing functional applications through enhanced cellular uptake, and might suppress unwanted off-target effects of siRNAs. NMR and X-ray crystallography studies showed that AM1 was an excellent mimic of phosphodiester linkages in RNA. The local conformational changes caused by the amide linkages were easily accommodated by small adjustments in RNA’s conformation. Further, the amide carbonyl group assumed an orientation that is similar to one of the nonbridging P–O bonds, which may enable amide/phosphate mimicry by conserving hydrogen bonding interactions. The crystal structure of a short amide-modified DNA-RNA hybrid in complex with RNase H indicated that the amide N–H could also act as an H-bond donor to stabilize RNA-protein interactions, which is an interaction mode not available to phosphate groups. Functional assays established that amides were well tolerated at internal positions in both strands of siRNAs. Surprisingly, amide modifications in the middle of the guide strand and at the 5′-end of the passenger strand increased RNAi activity compared to unmodified siRNA. Most importantly, an amide linkage between the first and second nucleosides of the passenger strand completely abolished its undesired off-target activity while enhancing the desired RNAi activity. These results suggest that RNAi may tolerate more substantial modifications of siRNAs than the chemistries tried so far. The findings are also important and timely because they demonstrate that amide modifications may reduce off-target activity of siRNAs, which remains an important roadblock for clinical use of RNAi. Taken together, our work suggests that amide linkages have underappreciated potential to optimize the biological and pharmacological properties of RNA. Expanded use of amide linkages in RNA to enhance CRISPR and other technologies requiring chemically stable, functional mimics of noncoding RNAs is expected.
中文翻译:

酰胺修饰的 RNA:使用蛋白质骨架调节短干扰 RNA 的功能。
基于 RNA 的控制基因表达的技术,如 RNA 干扰 (RNAi) 和 CRISPR-Cas9,已成为分子生物学和基因组学的强大工具。RNAi 和 CRISPR-Cas9 也可能成为新的治疗方法这一令人兴奋的潜力重新激发了人们对化学修饰 RNA 以改善其体内应用特性的兴趣。化学修饰可以提高酶稳定性、体内递送、细胞摄取和序列特异性,并最大限度地减少短干扰 RNA (siRNA) 和 CRISPR 相关 RNA 的脱靶活性。虽然已经出现了许多提高酶降解稳定性的良好解决方案,但后者功能特性的优化仍然具有挑战性。在这个帐户中,我们讨论了合成、结构、磷酸二酯骨架被酰胺键 (AM1) 取代的新型 RNA 非离子类似物的生物活性和生物活性。我们的长期目标是使用酰胺骨架来提高 siRNA 和其他功能性 RNA 的稳定性和特异性。我们在该领域的工作受到早期发现的推动,即非离子骨架修饰(包括 AM1)不会干扰 RNA 双链体的整体结构或热稳定性。我们假设 AM1 骨架修饰减少的负电荷和疏水性可能有助于通过增强细胞摄取来优化功能应用,并可能抑制 siRNA 的不必要的脱靶效应。NMR 和 X 射线晶体学研究表明,AM1 是 RNA 中磷酸二酯键的极好模拟物。由酰胺键引起的局部构象变化很容易通过 RNA 构象的微小调整来适应。此外,酰胺羰基的取向类似于非桥连 P-O 键之一,这可以通过保持氢键相互作用来模拟酰胺/磷酸酯。与 RNase H 复合的短酰胺修饰 DNA-RNA 杂交体的晶体结构表明,酰胺 N-H 还可以作为 H 键供体来稳定 RNA-蛋白质相互作用,这是磷酸盐无法使用的相互作用模式组。功能测定确定酰胺在两条 siRNA 链的内部位置都具有良好的耐受性。令人惊讶的是,与未修饰的 siRNA 相比,引导链中间和过客链 5' 端的酰胺修饰增加了 RNAi 活性。最重要的是,过客链的第一个和第二个核苷之间的酰胺键完全消除了其不需要的脱靶活性,同时增强了所需的 RNAi 活性。这些结果表明,与目前尝试的化学方法相比,RNAi 可以耐受更多的 siRNA 修饰。这些发现也很重要和及时,因为它们证明酰胺修饰可能会降低 siRNA 的脱靶活性,这仍然是 RNAi 临床应用的重要障碍。总之,我们的工作表明酰胺键在优化 RNA 的生物学和药理学特性方面的潜力没有得到充分重视。预计将扩大使用 RNA 中的酰胺键来增强 CRISPR 和其他需要化学稳定的非编码 RNA 功能模拟的技术。过客链的第一个和第二个核苷之间的酰胺键完全消除了其不需要的脱靶活性,同时增强了所需的 RNAi 活性。这些结果表明,与目前尝试的化学方法相比,RNAi 可以耐受更多的 siRNA 修饰。这些发现也很重要和及时,因为它们证明酰胺修饰可能会降低 siRNA 的脱靶活性,这仍然是 RNAi 临床应用的重要障碍。总之,我们的工作表明酰胺键在优化 RNA 的生物学和药理学特性方面的潜力没有得到充分重视。预计将扩大使用 RNA 中的酰胺键来增强 CRISPR 和其他需要化学稳定的非编码 RNA 功能模拟的技术。过客链的第一个和第二个核苷之间的酰胺键完全消除了其不需要的脱靶活性,同时增强了所需的 RNAi 活性。这些结果表明,与目前尝试的化学方法相比,RNAi 可以耐受更多的 siRNA 修饰。这些发现也很重要和及时,因为它们证明酰胺修饰可能会降低 siRNA 的脱靶活性,这仍然是 RNAi 临床应用的重要障碍。总之,我们的工作表明酰胺键在优化 RNA 的生物学和药理学特性方面的潜力没有得到充分重视。预计将扩大使用 RNA 中的酰胺键来增强 CRISPR 和其他需要化学稳定的非编码 RNA 功能模拟的技术。
更新日期:2020-09-15
中文翻译:

酰胺修饰的 RNA:使用蛋白质骨架调节短干扰 RNA 的功能。
基于 RNA 的控制基因表达的技术,如 RNA 干扰 (RNAi) 和 CRISPR-Cas9,已成为分子生物学和基因组学的强大工具。RNAi 和 CRISPR-Cas9 也可能成为新的治疗方法这一令人兴奋的潜力重新激发了人们对化学修饰 RNA 以改善其体内应用特性的兴趣。化学修饰可以提高酶稳定性、体内递送、细胞摄取和序列特异性,并最大限度地减少短干扰 RNA (siRNA) 和 CRISPR 相关 RNA 的脱靶活性。虽然已经出现了许多提高酶降解稳定性的良好解决方案,但后者功能特性的优化仍然具有挑战性。在这个帐户中,我们讨论了合成、结构、磷酸二酯骨架被酰胺键 (AM1) 取代的新型 RNA 非离子类似物的生物活性和生物活性。我们的长期目标是使用酰胺骨架来提高 siRNA 和其他功能性 RNA 的稳定性和特异性。我们在该领域的工作受到早期发现的推动,即非离子骨架修饰(包括 AM1)不会干扰 RNA 双链体的整体结构或热稳定性。我们假设 AM1 骨架修饰减少的负电荷和疏水性可能有助于通过增强细胞摄取来优化功能应用,并可能抑制 siRNA 的不必要的脱靶效应。NMR 和 X 射线晶体学研究表明,AM1 是 RNA 中磷酸二酯键的极好模拟物。由酰胺键引起的局部构象变化很容易通过 RNA 构象的微小调整来适应。此外,酰胺羰基的取向类似于非桥连 P-O 键之一,这可以通过保持氢键相互作用来模拟酰胺/磷酸酯。与 RNase H 复合的短酰胺修饰 DNA-RNA 杂交体的晶体结构表明,酰胺 N-H 还可以作为 H 键供体来稳定 RNA-蛋白质相互作用,这是磷酸盐无法使用的相互作用模式组。功能测定确定酰胺在两条 siRNA 链的内部位置都具有良好的耐受性。令人惊讶的是,与未修饰的 siRNA 相比,引导链中间和过客链 5' 端的酰胺修饰增加了 RNAi 活性。最重要的是,过客链的第一个和第二个核苷之间的酰胺键完全消除了其不需要的脱靶活性,同时增强了所需的 RNAi 活性。这些结果表明,与目前尝试的化学方法相比,RNAi 可以耐受更多的 siRNA 修饰。这些发现也很重要和及时,因为它们证明酰胺修饰可能会降低 siRNA 的脱靶活性,这仍然是 RNAi 临床应用的重要障碍。总之,我们的工作表明酰胺键在优化 RNA 的生物学和药理学特性方面的潜力没有得到充分重视。预计将扩大使用 RNA 中的酰胺键来增强 CRISPR 和其他需要化学稳定的非编码 RNA 功能模拟的技术。过客链的第一个和第二个核苷之间的酰胺键完全消除了其不需要的脱靶活性,同时增强了所需的 RNAi 活性。这些结果表明,与目前尝试的化学方法相比,RNAi 可以耐受更多的 siRNA 修饰。这些发现也很重要和及时,因为它们证明酰胺修饰可能会降低 siRNA 的脱靶活性,这仍然是 RNAi 临床应用的重要障碍。总之,我们的工作表明酰胺键在优化 RNA 的生物学和药理学特性方面的潜力没有得到充分重视。预计将扩大使用 RNA 中的酰胺键来增强 CRISPR 和其他需要化学稳定的非编码 RNA 功能模拟的技术。过客链的第一个和第二个核苷之间的酰胺键完全消除了其不需要的脱靶活性,同时增强了所需的 RNAi 活性。这些结果表明,与目前尝试的化学方法相比,RNAi 可以耐受更多的 siRNA 修饰。这些发现也很重要和及时,因为它们证明酰胺修饰可能会降低 siRNA 的脱靶活性,这仍然是 RNAi 临床应用的重要障碍。总之,我们的工作表明酰胺键在优化 RNA 的生物学和药理学特性方面的潜力没有得到充分重视。预计将扩大使用 RNA 中的酰胺键来增强 CRISPR 和其他需要化学稳定的非编码 RNA 功能模拟的技术。











































 京公网安备 11010802027423号
京公网安备 11010802027423号