当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the chemical space: Discovery of new anticancer 3‐arylbenzofuran derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-13 , DOI: 10.1002/jhet.4043 Jinhwang Kim 1 , Hyeon‐Min Cha 2 , Mikyung Park 3 , Dileep K. Singh 1 , Gi H. Bae 1 , Seong H. Kim 2, 3 , Ikyon Kim 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-13 , DOI: 10.1002/jhet.4043 Jinhwang Kim 1 , Hyeon‐Min Cha 2 , Mikyung Park 3 , Dileep K. Singh 1 , Gi H. Bae 1 , Seong H. Kim 2, 3 , Ikyon Kim 1
Affiliation
|
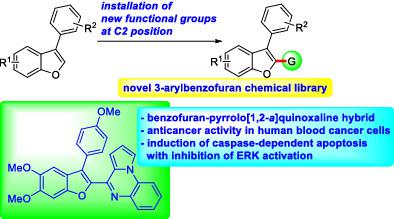
A new chemical space was generated via C2‐functionalization of 3‐arylbenzofurans. Mannich reaction of 3‐arylbenzofurans with secondary amines and formaldehyde allowed for installation of aminomethyl unit at C2 position of benzofurans. A formyl group at C2 site introduced as a result of Vilsmeier‐Haack formylation of 3‐arylbenzofurans was employed as a reacting partner for three‐component Kabachnik‐Fields reaction with various amines and triethyl phosphite to give a wide variety of aminomethylphosphonates. Furthermore, several benzo[d]oxazoles and pyrrolo[1,2‐a]quinoxalines were prepared by using the formyl group. Biological screening of the synthesized compounds revealed that the benzofuran bearing a pyrrolo[1,2‐a]quinoxaline moiety (5b) most potently inhibited the viability of human blood cancer cells, but not solid tumor cells. Caspase activity assay, analysis of Annexin V‐positive cells, and Western blot analysis indicated that 5b‐induced death of human lymphoma U937 cells could result from its potential to induce the caspase‐dependent apoptotic death of blood cancer cells with inhibition of ERK activation.
中文翻译:

扩大化学领域:发现新的抗癌3-芳基苯并呋喃衍生物
通过3-芳基苯并呋喃的C 2-官能化产生了一个新的化学空间。3-芳基苯并呋喃与仲胺和甲醛的曼尼希反应可将氨基甲基单元安装在苯并呋喃的C 2位。由Vilsmeier-Haack的3-芳基苯并呋喃甲酰化作用在C 2位引入的甲酰基被用作三组分Kabachnik-Fields与各种胺和亚磷酸三乙酯反应的反应伙伴,从而得到各种氨基甲基膦酸酯。此外,几种苯并[ d ]恶唑和吡咯并[1,2- a通过使用甲酰基制备]喹喔啉。对合成化合物的生物学筛选显示,带有吡咯并[1,2- a ]喹喔啉部分(5b)的苯并呋喃最能抑制人类血液癌细胞的活力,但不能抑制实体瘤细胞的活力。Caspase活性测定,膜联蛋白V阳性细胞分析和蛋白质印迹分析表明,5b诱导的人淋巴瘤U937细胞死亡可能是由于其具有通过抑制ERK激活来诱导血癌细胞caspase依赖性凋亡而导致的。
更新日期:2020-09-08
中文翻译:

扩大化学领域:发现新的抗癌3-芳基苯并呋喃衍生物
通过3-芳基苯并呋喃的C 2-官能化产生了一个新的化学空间。3-芳基苯并呋喃与仲胺和甲醛的曼尼希反应可将氨基甲基单元安装在苯并呋喃的C 2位。由Vilsmeier-Haack的3-芳基苯并呋喃甲酰化作用在C 2位引入的甲酰基被用作三组分Kabachnik-Fields与各种胺和亚磷酸三乙酯反应的反应伙伴,从而得到各种氨基甲基膦酸酯。此外,几种苯并[ d ]恶唑和吡咯并[1,2- a通过使用甲酰基制备]喹喔啉。对合成化合物的生物学筛选显示,带有吡咯并[1,2- a ]喹喔啉部分(5b)的苯并呋喃最能抑制人类血液癌细胞的活力,但不能抑制实体瘤细胞的活力。Caspase活性测定,膜联蛋白V阳性细胞分析和蛋白质印迹分析表明,5b诱导的人淋巴瘤U937细胞死亡可能是由于其具有通过抑制ERK激活来诱导血癌细胞caspase依赖性凋亡而导致的。










































 京公网安备 11010802027423号
京公网安备 11010802027423号