Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-07-13 , DOI: 10.1016/j.bmcl.2020.127401 Xia Chen 1 , Yao Dong 1 , Tianyue Guo 1 , Chao-Yie Yang 2 , Yong Chen 3 , Haiying Sun 1
|
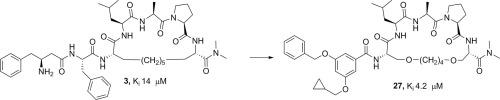
Telomeric repeat binding factor 2 (TRF2) plays an important role in protecting telomeres from being recognized as DNA breaks. TRF2 performs its telomere protecting functions partially by recruiting a number of accessory proteins to telomeres through its TRF homology (TFRH) domain. Identification of small molecular compounds which can bind to the TRFH domain of TRF2 and block the interactions between TRF2 and its associated proteins is crucial for elucidating the molecular mechanisms of these protein–protein interactions. Using a previously identified peptidic mimetic of ApolloTBM as a lead compound, we designed and synthesized a series of novel TRF2 inhibitors by non-peptidic modifications of the N-terminal residues. These compounds can maintain the binding affinities to TRF2 but have much reduced peptidic characteristics compared to the lead compound.
中文翻译:

ApolloTBM的N末端修饰的环肽模拟物作为TRF2抑制剂。
端粒重复序列结合因子2(TRF2)在保护端粒免于被DNA断裂识别中起重要作用。TRF2通过其TRF同源性(TFRH)域将大量辅助蛋白募集到端粒中,从而部分地发挥其端粒保护功能。鉴定可与TRF2的TRFH结构域结合并阻断TRF2及其相关蛋白之间相互作用的小分子化合物,对于阐明这些蛋白与蛋白相互作用的分子机制至关重要。使用先前鉴定的Apollo TBM肽模拟物作为先导化合物,我们通过N的非肽修饰设计并合成了一系列新型TRF2抑制剂-末端残基。这些化合物可以保持与TRF2的结合亲和力,但与前导化合物相比,其肽段特性大大降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号