当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of the usher chaperone YadV reveals a monomer with the proline lock in closed conformation suggestive of an intermediate state
FEBS Letters ( IF 3.5 ) Pub Date : 2020-07-23 , DOI: 10.1002/1873-3468.13883 Nishant Kumar Pandey 1, 2 , Garima Verma 1 , Gajraj Singh Kushwaha 1 , Mrutyunjay Suar 2 , Neel Sarovar Bhavesh 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-07-23 , DOI: 10.1002/1873-3468.13883 Nishant Kumar Pandey 1, 2 , Garima Verma 1 , Gajraj Singh Kushwaha 1 , Mrutyunjay Suar 2 , Neel Sarovar Bhavesh 1
Affiliation
|
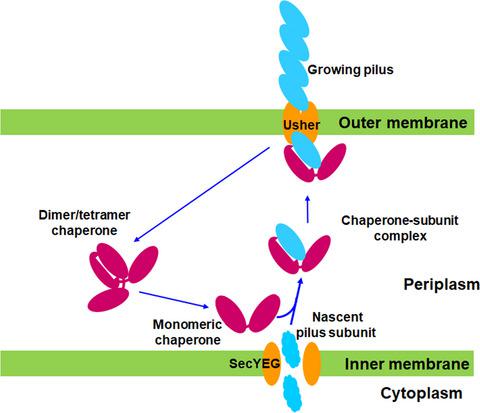
Cell surface pili assembled by the chaperone–usher (CU) pathway play a crucial role in the adhesion of uropathogenic Escherichia coli. YadV is the chaperone component of the CU pathway of Yad pili. Here, we report the crystal structure of YadV from E. coli. In contrast to major usher chaperones, YadV is a monomer in solution as well as in the crystallographic symmetry, and the monomeric form is a preferred state for interacting with pilus subunits. Moreover, we observed a closed conformation for the proline lock, a crucial structural element for chaperone–pilus subunit interaction. MD simulation shows that the closed state of the proline lock is not energetically stable. Thus, the structure of monomeric YadV with its closed proline lock may serve as an intermediate state to provide suitable access to pilus subunits.
中文翻译:

引导分子伴侣 YadV 的晶体结构揭示了一个具有闭合构象的脯氨酸锁的单体,表明处于中间状态
由伴侣-引导(CU)途径组装的细胞表面菌毛在尿路致病性大肠杆菌的粘附中起着至关重要的作用。YadV 是 Yad pili CU 通路的伴侣成分。在这里,我们报告了来自大肠杆菌的 YadV 的晶体结构。与主要的引导分子伴侣相比,YadV 是溶液中的单体以及晶体对称性,单体形式是与菌毛亚基相互作用的首选状态。此外,我们观察到脯氨酸锁的闭合构象,这是分子伴侣-菌毛亚基相互作用的关键结构元素。MD 模拟表明脯氨酸锁的关闭状态不是能量稳定的。因此,具有闭合脯氨酸锁的单体 YadV 的结构可以作为中间状态,以提供对菌毛亚基的合适通路。
更新日期:2020-07-23
中文翻译:

引导分子伴侣 YadV 的晶体结构揭示了一个具有闭合构象的脯氨酸锁的单体,表明处于中间状态
由伴侣-引导(CU)途径组装的细胞表面菌毛在尿路致病性大肠杆菌的粘附中起着至关重要的作用。YadV 是 Yad pili CU 通路的伴侣成分。在这里,我们报告了来自大肠杆菌的 YadV 的晶体结构。与主要的引导分子伴侣相比,YadV 是溶液中的单体以及晶体对称性,单体形式是与菌毛亚基相互作用的首选状态。此外,我们观察到脯氨酸锁的闭合构象,这是分子伴侣-菌毛亚基相互作用的关键结构元素。MD 模拟表明脯氨酸锁的关闭状态不是能量稳定的。因此,具有闭合脯氨酸锁的单体 YadV 的结构可以作为中间状态,以提供对菌毛亚基的合适通路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号