Water Research ( IF 11.4 ) Pub Date : 2020-07-12 , DOI: 10.1016/j.watres.2020.116172 Min Sik Kim 1 , Ki-Myeong Lee 2 , Hak-Hyeon Kim 3 , Hongshin Lee 4 , Dae Won Kim 5 , Jae-Hong Kim 6 , Changha Lee 2
|
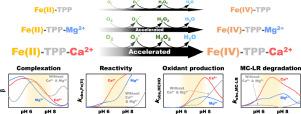
Fe(II)-tetrapolyphosphate complexes are known to activate molecular oxygen (Fe(II)-TPP/O2) to produce reactive oxidants (most likely, Fe(IV)-TPP complexes) that are capable of degrading refractory organic contaminants in water. This study found that magnesium and calcium ions (Mg2+ and Ca2+) accelerate the degradation of micfrocystin-LR (MC-LR), the most toxic and abundant cyanotoxin, by the Fe(II)-TPP/O2 system. The addition of Mg2+ and Ca2+ increased the observed rate constant of MC-LR degradation by up to 4.3 and 14.8 folds, respectively. Mg2+ and Ca2+ accelerated the MC-LR degradation in the entire pH range, except for the alkaline region with pH > ca. 10. The addition of Mg2+ and Ca2+ also reshaped the pH-dependency of the MC-LR degradation, greatly increasing the rate of MC-LR degradation at neutral pH. It was found that Mg2+ and Ca2+ accelerate the reaction of Fe(II)-TPP complexes with oxygen, resulting in faster production of reactive oxidants. The findings from cyclic voltammetry and potentiometric titration suggest that Mg2+ and Ca2+ form ternary complexes with Fe(II)-TPP, which exhibit higher reactivity with oxygen. Due to the effects of Mg2+ and Ca2+, the rate of MC-LR degradation by the Fe(II)-TPP/O2 system was even higher in natural water than in deionized water.
中文翻译:

在镁和钙离子存在下,Fe(II)-四聚磷酸盐/氧加速微囊藻毒素-LR的氧化。
已知Fe(II)-四聚磷酸盐复合物可活化分子氧(Fe(II)-TPP / O 2)产生能降解水中难降解有机污染物的活性氧化剂(极有可能是Fe(IV)-TPP复合物) 。这项研究发现,镁和钙离子(Mg 2+和Ca 2+)可通过Fe(II)-TPP / O 2系统加速最具毒性和最丰富的蓝藻毒素微丝藻毒素(MC-LR)的降解。Mg 2+和Ca 2+的添加使观察到的MC-LR降解速率常数分别增加了4.3倍和14.8倍。Mg 2+和Ca 2+在整个pH范围内加速了MC-LR的降解,除了pH> ca的碱性区域。10. Mg 2+和Ca 2+的添加还改变了MC-LR降解的pH依赖性,从而大大提高了中性pH时MC-LR的降解速率。发现Mg 2+和Ca 2+加速Fe(II)-TPP配合物与氧气的反应,导致更快地产生反应性氧化剂。循环伏安法和电位滴定法的发现表明,Mg 2+和Ca 2+与Fe(II)-TPP形成三元络合物,与氧的反应性更高。由于Mg 2+和Ca 2+的影响,Fe(II)-TPP / O 2系统在自然水中的MC-LR降解速率甚至比去离子水中更高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号