Cellular Signalling ( IF 4.4 ) Pub Date : 2020-07-11 , DOI: 10.1016/j.cellsig.2020.109712 Zhaojia Wu 1 , Parker L Andersen 2 , Trevor Moraes 3 , Sean A McKenna 4 , Yiran Zhang 5 , Weiwei Zhang 5 , Michael J Ellison 6 , Wei Xiao 1
|
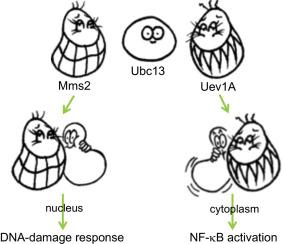
The ubiquitin (Ub)-conjugating enzyme variants (Uev) Uev1A and Mms2 interact with Ubc13 to form heterodimeric complexes with different biological functions. Uev1A-Ubc13 is involved in NF-κB activation while Mms2-Ubc13 is required for the DNA-damage response. The structural comparison of the core domains of these two Uevs reveals no obvious difference, suggesting that the amino terminal extension of Uev1A plays a critical role in the functional determination. Indeed, truncated Uev1A lacking the N-terminal extension behaves like Mms2, while a chimeric protein containing the N-terminal Uev1A fused to Mms2 functionally resembles Uev1A. Interestingly, the N-terminal extension of Uev1A also dictates whether to assemble di- or poly-Ub chains in an in vitro reaction. Both thermodynamic measurements and enzymatic assays revealed that the Uev1A N-terminal extension weakens the Uev-Ubc13 interaction; however, other means capable of causing a reduced Uev1A-Ubc13 affinity and poly-Ub chain assembly do not necessarily promote NF-κB activation, indicating that the poly-Ub chain formation is not the only component contributed by the N-terminal extension of Uev1A. The physiological relevance of the Uev1A N-terminal truncation is presented and discussed.
中文翻译:

Uev1A 氨基末端刺激多泛素链组装,是 NF-κB 激活所必需的。
泛素 (Ub) 结合酶变体 (Uev) Uev1A 和 Mms2 与 Ubc13 相互作用,形成具有不同生物学功能的异二聚体复合物。Uev1A-Ubc13 参与 NF-κB 激活,而 Mms2-Ubc13 是 DNA 损伤反应所必需的。这两个Uevs核心域的结构比较没有发现明显差异,表明Uev1A的氨基末端延伸在功能决定中起着关键作用。事实上,缺乏 N 端延伸的截短 Uev1A 的行为类似于 Mms2,而含有与 Mms2 融合的 N 端 Uev1A 的嵌合蛋白在功能上类似于 Uev1A。有趣的是,Uev1A 的 N 端延伸也决定了在体外组装双 Ub 链还是多 Ub 链。反应。热力学测量和酶促测定均表明 Uev1A N 端延伸削弱了 Uev-Ubc13 相互作用;然而,其他能够导致 Uev1A-Ubc13 亲和力降低和聚 Ub 链组装的方法不一定会促进 NF-κB 激活,这表明聚 Ub 链的形成并不是 Uev1A N 端延伸的唯一组成部分. 介绍和讨论了 Uev1A N 端截断的生理相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号