当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Advanced electrochemical oxidation for the simultaneous removal of manganese and generation of permanganate oxidant
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-07-10 , DOI: 10.1039/d0ew00261e Sean T. McBeath 1, 2, 3, 4 , David P. Wilkinson 5, 6, 7, 8 , Nigel J. D. Graham 1, 2, 3, 4
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-07-10 , DOI: 10.1039/d0ew00261e Sean T. McBeath 1, 2, 3, 4 , David P. Wilkinson 5, 6, 7, 8 , Nigel J. D. Graham 1, 2, 3, 4
Affiliation
|
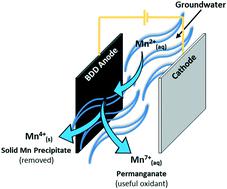
Emerging electrochemical systems, such as advanced electro-oxidation, provide a potentially powerful alternative to conventional oxidation processes which can often be unsuitable for small, remote and decentralised system applications. The one electro-oxidation process, which may be well suited for these applications, is the use of high oxygen overpotential boron-doped diamond (BDD) electrodes, as a pre-oxidation step for the removal of various raw water contaminants. While BDD electro-oxidation has been studied extensively for the abatement of organic micropollutants, its application as a pre-oxidation technology for the removal of soluble manganese (Mn2+) in source waters for drinking water supply, has not been reported to-date. In this study, we summarise the results of tests using a bench-scale electro-oxidation system and synthetic Mn2+ solutions in order to consider the simultaneous removal of manganese and the generation of permanganate. The results showed that total manganese was reduced by 9.1, 38.7 and 57.4% at current densities of 10, 40 and 80 mA cm−2, respectively, with an initial Mn2+ concentration of 39 μM. Increased Mn removal at higher current density was attributed to increased generation of, and reaction with, hydroxyl radicals, indicated by a direct proportional relationship between pseudo-first order reaction rate constants for methanol (˙OH radical scavenger) and current density. A mathematical model was developed to describe Mn removal under mass transport limitations, and was found to correlate well with experimental data. Finally, a completely novel synthesis pathway for the generation of permanganate species (Mn7+) is presented, whereby concentrations up to 0.9 μM were synthesised from Mn2+ in circumneutral conditions.
中文翻译:

先进的电化学氧化技术,可同时去除锰并生成高锰酸盐氧化剂
新兴的电化学系统,例如高级电氧化,为常规氧化工艺提供了潜在的强大替代方法,而传统的氧化工艺通常不适合小型,偏远和分散的系统应用。一种可能非常适合这些应用的电氧化工艺是使用高氧超电势的掺硼金刚石(BDD)电极,作为去除各种原水污染物的预氧化步骤。尽管对BDD电氧化技术进行了广泛的研究以减少有机微量污染物,但其作为一种预氧化技术用于去除可溶性锰(Mn 2+)至今尚未报道用于饮用水源的水。在这项研究中,我们总结了使用台式规模的电氧化系统和合成Mn 2+溶液的测试结果,以考虑同时去除锰和生成高锰酸盐。结果表明,当初始电流为Mn 2+时,在电流密度分别为10、40和80 mA cm -2时,总锰分别减少9.1%,38.7%和57.4%。浓度为39μM。在较高电流密度下锰去除量的增加归因于羟基自由基的生成和与羟基自由基的反应增加,这通过甲醇(methanolOH自由基清除剂)的拟一级反应速率常数与电流密度之间的直接比例关系表明。建立了一个数学模型来描述在传质限制下的锰去除,并发现它与实验数据很好地相关。最后,提出了一种用于生成高锰酸盐物种(Mn 7+)的全新途径,从而在环境条件下从Mn 2+合成了高达0.9μM的浓度。
更新日期:2020-08-27
中文翻译:

先进的电化学氧化技术,可同时去除锰并生成高锰酸盐氧化剂
新兴的电化学系统,例如高级电氧化,为常规氧化工艺提供了潜在的强大替代方法,而传统的氧化工艺通常不适合小型,偏远和分散的系统应用。一种可能非常适合这些应用的电氧化工艺是使用高氧超电势的掺硼金刚石(BDD)电极,作为去除各种原水污染物的预氧化步骤。尽管对BDD电氧化技术进行了广泛的研究以减少有机微量污染物,但其作为一种预氧化技术用于去除可溶性锰(Mn 2+)至今尚未报道用于饮用水源的水。在这项研究中,我们总结了使用台式规模的电氧化系统和合成Mn 2+溶液的测试结果,以考虑同时去除锰和生成高锰酸盐。结果表明,当初始电流为Mn 2+时,在电流密度分别为10、40和80 mA cm -2时,总锰分别减少9.1%,38.7%和57.4%。浓度为39μM。在较高电流密度下锰去除量的增加归因于羟基自由基的生成和与羟基自由基的反应增加,这通过甲醇(methanolOH自由基清除剂)的拟一级反应速率常数与电流密度之间的直接比例关系表明。建立了一个数学模型来描述在传质限制下的锰去除,并发现它与实验数据很好地相关。最后,提出了一种用于生成高锰酸盐物种(Mn 7+)的全新途径,从而在环境条件下从Mn 2+合成了高达0.9μM的浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号