当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-Catalyzed Cross-Couplings between Alkyl Halides and Grignard Reagents.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.accounts.0c00238 Amandine Guérinot 1 , Janine Cossy 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.accounts.0c00238 Amandine Guérinot 1 , Janine Cossy 1
Affiliation
|
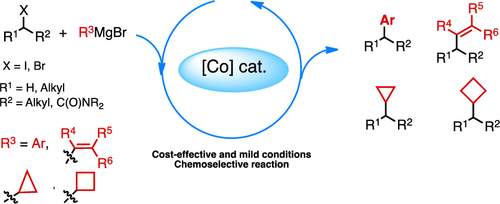
Metal-catalyzed cross-couplings have emerged as essential tools for the construction of C–C bonds. The identification of efficient catalytic systems as well as large substrate scope made these cross-couplings key reactions to access valuable molecules ranging from materials, agrochemicals to active pharmaceutical ingredients. They have been increasingly integrated in retrosynthetic plans, allowing shorter and original route development. Palladium-catalyzed cross-couplings still largely rule the field, with the most popular reactions in industrial processes being the Suzuki and Sonogashira couplings. However, the extensive use of palladium complexes raises several problems such as limited resources, high cost, environmental impact, and frequent need for sophisticated ligands. As a consequence, the use of nonprecious and cheap metal catalysts has appeared as a new horizon in cross-coupling development. Over the last three decades, a growing interest has thus been devoted to Fe-, Co-, Cu-, or Ni-catalyzed cross-couplings. Their natural abundance makes them cost-effective, allowing the conception of more sustainable and less expensive chemical processes, especially for large-scale production of active molecules. In addition to these economical and environmental considerations, the 3d metal catalysts also exhibit complementary reactivity with palladium complexes, facilitating the use of alkyl halide partners due to the decrease of β-elimination side reactions. In particular, by using cobalt catalysts, numerous cross-couplings between alkyl halides and organometallics have been described. However, cobalt catalysis still stays far behind palladium catalysis in terms of popularity and applications, and the expansion of the substrate scope as well as the development of simple and robust catalytic systems remains an important challenge.
中文翻译:

烷基卤化物与格氏试剂之间的钴催化交叉偶联。
金属催化的交叉偶联已成为构建C-C键的重要工具。高效催化系统的识别以及较大的底物范围使这些交叉偶联成为关键反应,可访问从材料,农用化学品到活性药物成分等有价值的分子。它们已越来越多地集成在逆合成计划中,从而可以开发更短且更原始的路线。钯催化的交叉偶联仍在很大程度上控制着该领域,在工业过程中最流行的反应是铃木和Sonogashira偶联。然而,钯配合物的广泛使用引起了一些问题,例如资源有限,成本高,对环境的影响以及对复杂配体的频繁需求。作为结果,非贵金属和廉价金属催化剂的使用已成为交叉偶联发展的新视野。因此,在过去的三十年中,人们越来越关注铁,钴,铜或镍催化的交叉偶联。它们的天然丰度使其具有成本效益,从而可以构想更可持续,更便宜的化学过程,尤其是对于大规模生产活性分子而言。除了这些经济和环境方面的考虑外,3d金属催化剂还表现出与钯配合物的互补反应性,由于减少了β-消除副反应,因此便于使用卤代烷伙伴。特别是,通过使用钴催化剂,已经描述了卤代烷和有机金属之间的许多交叉偶联。然而,
更新日期:2020-07-21
中文翻译:

烷基卤化物与格氏试剂之间的钴催化交叉偶联。
金属催化的交叉偶联已成为构建C-C键的重要工具。高效催化系统的识别以及较大的底物范围使这些交叉偶联成为关键反应,可访问从材料,农用化学品到活性药物成分等有价值的分子。它们已越来越多地集成在逆合成计划中,从而可以开发更短且更原始的路线。钯催化的交叉偶联仍在很大程度上控制着该领域,在工业过程中最流行的反应是铃木和Sonogashira偶联。然而,钯配合物的广泛使用引起了一些问题,例如资源有限,成本高,对环境的影响以及对复杂配体的频繁需求。作为结果,非贵金属和廉价金属催化剂的使用已成为交叉偶联发展的新视野。因此,在过去的三十年中,人们越来越关注铁,钴,铜或镍催化的交叉偶联。它们的天然丰度使其具有成本效益,从而可以构想更可持续,更便宜的化学过程,尤其是对于大规模生产活性分子而言。除了这些经济和环境方面的考虑外,3d金属催化剂还表现出与钯配合物的互补反应性,由于减少了β-消除副反应,因此便于使用卤代烷伙伴。特别是,通过使用钴催化剂,已经描述了卤代烷和有机金属之间的许多交叉偶联。然而,











































 京公网安备 11010802027423号
京公网安备 11010802027423号