当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orthogonal Stability and Reactivity of Aryl Germanes Enables Rapid and Selective (Multi)Halogenations.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-12 , DOI: 10.1002/anie.202008372 Christoph Fricke 1 , Kristina Deckers 1 , Franziska Schoenebeck 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-12 , DOI: 10.1002/anie.202008372 Christoph Fricke 1 , Kristina Deckers 1 , Franziska Schoenebeck 1
Affiliation
|
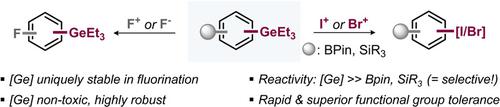
While halogenation is of key importance in synthesis and radioimaging, the currently available repertoire is largely designed to introduce a single halogen per molecule. This report makes the selective introduction of several different halogens accessible. Showcased here is the privileged stability of nontoxic aryl germanes under harsh fluorination conditions (that allow selective fluorination in their presence), while displaying superior reactivity and functional‐group tolerance in electrophilic iodinations and brominations, outcompeting silanes or boronic esters under rapid and additive‐free conditions. Mechanistic experiments and computational studies suggest a concerted electrophilic aromatic substitution as the underlying mechanism.
中文翻译:

芳基锗烷的正交稳定性和反应性可实现快速、选择性(多)卤化。
虽然卤化在合成和放射成像中至关重要,但目前可用的方法主要是为每个分子引入一个卤素。该报告使得可以选择性地引入几种不同的卤素。这里展示的是无毒芳基锗烷在严酷的氟化条件下(允许在其存在的情况下进行选择性氟化)的优越稳定性,同时在亲电碘化和溴化中表现出优异的反应活性和官能团耐受性,在快速且无添加剂的情况下超越硅烷或硼酸酯状况。机理实验和计算研究表明协同亲电芳香取代是潜在的机制。
更新日期:2020-07-12
中文翻译:

芳基锗烷的正交稳定性和反应性可实现快速、选择性(多)卤化。
虽然卤化在合成和放射成像中至关重要,但目前可用的方法主要是为每个分子引入一个卤素。该报告使得可以选择性地引入几种不同的卤素。这里展示的是无毒芳基锗烷在严酷的氟化条件下(允许在其存在的情况下进行选择性氟化)的优越稳定性,同时在亲电碘化和溴化中表现出优异的反应活性和官能团耐受性,在快速且无添加剂的情况下超越硅烷或硼酸酯状况。机理实验和计算研究表明协同亲电芳香取代是潜在的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号