Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetic core–shell Fe3O4@Cu2O and Fe3O4@Cu2O–Cu materials as catalysts for aerobic oxidation of benzylic alcohols assisted by TEMPO and N-methylimidazole
RSC Advances ( IF 3.9 ) Pub Date : 2020-7-10 , DOI: 10.1039/d0ra04064a Binyu Xu 1 , Samuthirarajan Senthilkumar 2 , Wei Zhong 2 , Zhongquan Shen 2 , Chunxin Lu 2 , Xiaoming Liu 1, 2
RSC Advances ( IF 3.9 ) Pub Date : 2020-7-10 , DOI: 10.1039/d0ra04064a Binyu Xu 1 , Samuthirarajan Senthilkumar 2 , Wei Zhong 2 , Zhongquan Shen 2 , Chunxin Lu 2 , Xiaoming Liu 1, 2
Affiliation
|
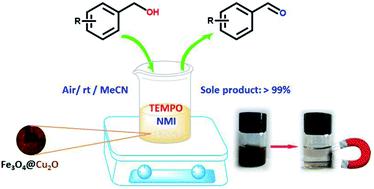
In this work, core–shell Fe3O4@Cu2O and Fe3O4@Cu2O–Cu nanomaterials for aerobic oxidation of benzylic alcohols are reported with 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) and N-methylimidazole (NMI) as the co-catalysts. To anchor Cu2O nanoparticles around the magnetic particles under solvothermal conditions, the magnetic material Fe3O4 was modified by grafting a layer of L-lysine (L-Lys) to introduce –NH2 groups at the surface of the magnetic particles. With amine groups as the anchor, Cu(NO3)2 was used to co-precipitate the desired Cu2O by using ethylene glycol as the reducing agent. Prolonging the reaction time would lead to over-reduced forms of the magnetic materials in the presence of copper, Fe3O4@Cu2O–Cu. The nanomaterials and its precursors were fully characterized by a variety of spectroscopic techniques. In combination with both TEMPO and NMI, these materials showed excellent catalytic activities in aerobic oxidation of benzylic alcohols under ambient conditions. For most of the benzylic alcohols, the conversion into aldehydes was nearly quantitative with aldehydes as the sole product. The materials were recyclable and robust. Up to 7 repeat runs, its activity dropped less than 10%. The over-reduced materials, Fe3O4@Cu2O–Cu, exhibited slightly better performance in durability. The magnetic properties allowed easy separation after reaction by simply applying an external magnet.
中文翻译:

磁性核壳 Fe3O4@Cu2O 和 Fe3O4@Cu2O-Cu 材料作为 TEMPO 和 N-甲基咪唑辅助有氧氧化苯甲醇的催化剂
在这项工作中,以2,2,6,6-四甲基哌啶-N-氧基(_ TEMPO) 和N-甲基咪唑 (NMI) 作为助催化剂。为了在溶剂热条件下将 Cu 2 O 纳米颗粒固定在磁性颗粒周围,通过接枝 L-赖氨酸 ( L -Lys) 层引入-NH 2对磁性材料Fe 3 O 4进行改性。磁性粒子表面的基团。以胺基为锚,以乙二醇为还原剂,以Cu(NO 3 ) 2共沉淀出所需的Cu 2 O。延长反应时间会导致在铜、Fe 3 O 4 @Cu 2存在下磁性材料的过度还原形式O-铜。纳米材料及其前体通过各种光谱技术进行了充分表征。与 TEMPO 和 NMI 结合使用,这些材料在环境条件下对苯甲醇的有氧氧化表现出优异的催化活性。对于大多数苄醇,醛类的转化几乎是定量的,醛类是唯一的产物。这些材料可回收且坚固。多达 7 次重复运行,其活动下降不到 10%。过度还原的材料 Fe 3 O 4 @Cu 2 O–Cu 在耐久性方面表现出稍好的性能。通过简单地施加外部磁体,磁性可以在反应后轻松分离。
更新日期:2020-07-10
中文翻译:

磁性核壳 Fe3O4@Cu2O 和 Fe3O4@Cu2O-Cu 材料作为 TEMPO 和 N-甲基咪唑辅助有氧氧化苯甲醇的催化剂
在这项工作中,以2,2,6,6-四甲基哌啶-N-氧基(_ TEMPO) 和N-甲基咪唑 (NMI) 作为助催化剂。为了在溶剂热条件下将 Cu 2 O 纳米颗粒固定在磁性颗粒周围,通过接枝 L-赖氨酸 ( L -Lys) 层引入-NH 2对磁性材料Fe 3 O 4进行改性。磁性粒子表面的基团。以胺基为锚,以乙二醇为还原剂,以Cu(NO 3 ) 2共沉淀出所需的Cu 2 O。延长反应时间会导致在铜、Fe 3 O 4 @Cu 2存在下磁性材料的过度还原形式O-铜。纳米材料及其前体通过各种光谱技术进行了充分表征。与 TEMPO 和 NMI 结合使用,这些材料在环境条件下对苯甲醇的有氧氧化表现出优异的催化活性。对于大多数苄醇,醛类的转化几乎是定量的,醛类是唯一的产物。这些材料可回收且坚固。多达 7 次重复运行,其活动下降不到 10%。过度还原的材料 Fe 3 O 4 @Cu 2 O–Cu 在耐久性方面表现出稍好的性能。通过简单地施加外部磁体,磁性可以在反应后轻松分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号