当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A DFT study for CO2 hydrogenation on W(111) and Ni-doped W(111) surfaces.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-10 , DOI: 10.1039/d0cp02285c Minhua Zhang 1 , Song Yin 1 , Yifei Chen 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-10 , DOI: 10.1039/d0cp02285c Minhua Zhang 1 , Song Yin 1 , Yifei Chen 1
Affiliation
|
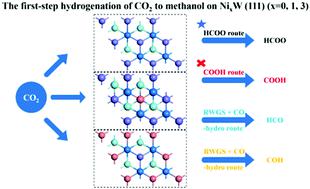
The first-step hydrogenation of CO2 to methanol via a HCOO route, COOH route, and RWGS + CO-hydro route on NixW(111) (x = 0, 1, 3) has been studied using density functional theory (DFT) calculations. CO2 and H could be chemically adsorbed on Ni-doped W(111) surfaces with relatively high adsorption energy, due to the synergistic effect of W that helps anchoring CO2 and Ni that facilitates the adsorption of H. The HCOO route is the main path for the first-step hydrogenation of CO2 with lower barriers on all three surfaces. Besides, competition between the HCOO route and RWGS + CO-hydro route could be enhanced with the increase in doped Ni on the W(111) surface. Furthermore, the first-step hydrogenation of CO2 hardly undergoes the COOH pathway because of the higher barriers, although the doping of Ni has slightly reduced the barrier of COOH formation. Our calculated results indicate that the W(111) and Ni-doped W(111) surface are potential candidate surfaces for CO2 hydrogenation to methanol, and Ni doping could influence the selectivity of reduction pathways.
中文翻译:

DFT研究W(111)和掺Ni的W(111)表面上的CO2加氢。
使用密度泛函理论(DFT)研究了通过HCOO路线,COOH路线和RWGS + CO-氢路线在Ni x W(111)(x = 0,1,3)上将CO 2第一步加氢成甲醇)计算。由于W的协同作用有助于锚定CO 2和Ni促进H的吸附,因此CO 2和H可以化学方式吸附在具有较高吸附能的Ni掺杂的W(111)表面上。HCOO途径是主要的第一步CO 2加氢的路径在所有三个表面上都具有较低的壁垒。此外,随着W(111)表面掺杂Ni含量的增加,HCOO路线与RWGS + CO-水力路线之间的竞争可能会加剧。此外,尽管Ni的掺杂略微降低了COOH形成的障碍,但由于较高的势垒,CO 2的第一步氢化几乎不经历COOH途径。我们的计算结果表明,W(111)和掺Ni的W(111)表面是CO 2加氢制甲醇的潜在候选表面,而Ni掺杂可能会影响还原途径的选择性。
更新日期:2020-08-05
中文翻译:

DFT研究W(111)和掺Ni的W(111)表面上的CO2加氢。
使用密度泛函理论(DFT)研究了通过HCOO路线,COOH路线和RWGS + CO-氢路线在Ni x W(111)(x = 0,1,3)上将CO 2第一步加氢成甲醇)计算。由于W的协同作用有助于锚定CO 2和Ni促进H的吸附,因此CO 2和H可以化学方式吸附在具有较高吸附能的Ni掺杂的W(111)表面上。HCOO途径是主要的第一步CO 2加氢的路径在所有三个表面上都具有较低的壁垒。此外,随着W(111)表面掺杂Ni含量的增加,HCOO路线与RWGS + CO-水力路线之间的竞争可能会加剧。此外,尽管Ni的掺杂略微降低了COOH形成的障碍,但由于较高的势垒,CO 2的第一步氢化几乎不经历COOH途径。我们的计算结果表明,W(111)和掺Ni的W(111)表面是CO 2加氢制甲醇的潜在候选表面,而Ni掺杂可能会影响还原途径的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号