当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Impact of Surfactant Type and Digestion: Highly Digestible Surfactants Improve Oral Bioavailability of Nilotinib.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.molpharmaceut.0c00305 Niklas J Koehl 1 , René Holm 2, 3 , Martin Kuentz 4 , Vincent Jannin 5 , Brendan T Griffin 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.molpharmaceut.0c00305 Niklas J Koehl 1 , René Holm 2, 3 , Martin Kuentz 4 , Vincent Jannin 5 , Brendan T Griffin 1
Affiliation
|
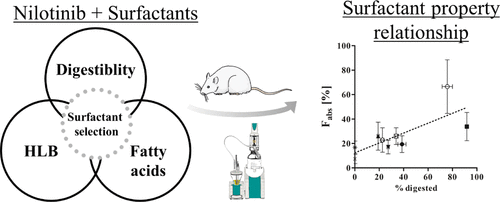
The scientific rationale for selection of the surfactant type during oral formulation development requires an in-depth understanding of the interplay between surfactant characteristics and biopharmaceutical factors. Currently, however, there is a lack of comprehensive knowledge of how surfactant properties, such as hydrophilic–lipophilic balance (HLB), digestibility, and fatty acid (FA) chain length, translate into in vivo performance. In the present study, the relationship between surfactant properties, in vitro characteristics, and in vivo bioavailability was systematically evaluated. An in vitro lipolysis model was used to study the digestibility of a variety of nonionic surfactants. Eight surfactants and one surfactant mixture were selected for further analysis using the model poorly water-soluble drug nilotinib. In vitro lipolysis of all nilotinib formulations was performed, followed by an in vivo pharmacokinetic evaluation in rats. The in vitro lipolysis studies showed that medium-chain FA-based surfactants were more readily digested compared to long-chain surfactants. The in vivo study demonstrated that a Tween 20 formulation significantly enhanced the absolute bioavailability of nilotinib up to 5.2-fold relative to an aqueous suspension. In general, surfactants that were highly digestible in vitro tended to display higher bioavailability of nilotinib in vivo. The bioavailability may additionally be related to the FA chain length of digestible surfactants with an improved exposure in the case of medium-chain FA-based surfactants. There was no apparent relationship between the HLB value of surfactants and the in vivo bioavailability of nilotinib. The impact of this study’s findings suggests that when designing surfactant-based formulations to enhance oral bioavailability of the poorly water-soluble drug nilotinib, highly digestible, medium chain-based surfactants are preferred. Additionally, for low-permeability drugs such as nilotinib, which is subject to efflux by intestinal P-glycoprotein, the biopharmaceutical effects of surfactants merit further consideration.
中文翻译:

探索表面活性剂类型和消化的影响:高度易消化的表面活性剂提高尼罗替尼的口服生物利用度。
在口服制剂开发过程中选择表面活性剂类型的科学原理需要深入了解表面活性剂特性和生物制药因素之间的相互作用。然而,目前缺乏关于表面活性剂特性如何转化为体内性能的全面知识,例如亲水-亲油平衡 (HLB)、消化率和脂肪酸 (FA) 链长。在本研究中,系统地评估了表面活性剂特性、体外特性和体内生物利用度之间的关系。体外_脂肪分解模型用于研究多种非离子表面活性剂的消化率。选择八种表面活性剂和一种表面活性剂混合物使用模型难溶于水的药物尼罗替尼进行进一步分析。对所有尼罗替尼制剂进行体外脂解,然后在大鼠中进行体内药代动力学评估。体外脂肪分解研究表明,与长链表面活性剂相比,基于中链 FA 的表面活性剂更容易消化。体内研究表明,相对于水性悬浮液,Tween 20 制剂可显着提高尼罗替尼的绝对生物利用度高达 5.2 倍。一般来说,在体外高度易消化的表面活性剂倾向于在体内显示出更高的尼罗替尼生物利用度。生物利用度可能还与可消化表面活性剂的 FA 链长有关,在中链 FA 基表面活性剂的情况下暴露量有所提高。表面活性剂的HLB值与尼罗替尼的体内生物利用度之间没有明显的关系。本研究结果的影响表明,在设计基于表面活性剂的制剂以提高难溶于水的药物尼罗替尼的口服生物利用度时,优选高度易消化的中链表面活性剂。此外,对于低渗透性药物,如尼罗替尼,易被肠道P-糖蛋白排出,表面活性剂的生物药学作用值得进一步考虑。
更新日期:2020-09-09
中文翻译:

探索表面活性剂类型和消化的影响:高度易消化的表面活性剂提高尼罗替尼的口服生物利用度。
在口服制剂开发过程中选择表面活性剂类型的科学原理需要深入了解表面活性剂特性和生物制药因素之间的相互作用。然而,目前缺乏关于表面活性剂特性如何转化为体内性能的全面知识,例如亲水-亲油平衡 (HLB)、消化率和脂肪酸 (FA) 链长。在本研究中,系统地评估了表面活性剂特性、体外特性和体内生物利用度之间的关系。体外_脂肪分解模型用于研究多种非离子表面活性剂的消化率。选择八种表面活性剂和一种表面活性剂混合物使用模型难溶于水的药物尼罗替尼进行进一步分析。对所有尼罗替尼制剂进行体外脂解,然后在大鼠中进行体内药代动力学评估。体外脂肪分解研究表明,与长链表面活性剂相比,基于中链 FA 的表面活性剂更容易消化。体内研究表明,相对于水性悬浮液,Tween 20 制剂可显着提高尼罗替尼的绝对生物利用度高达 5.2 倍。一般来说,在体外高度易消化的表面活性剂倾向于在体内显示出更高的尼罗替尼生物利用度。生物利用度可能还与可消化表面活性剂的 FA 链长有关,在中链 FA 基表面活性剂的情况下暴露量有所提高。表面活性剂的HLB值与尼罗替尼的体内生物利用度之间没有明显的关系。本研究结果的影响表明,在设计基于表面活性剂的制剂以提高难溶于水的药物尼罗替尼的口服生物利用度时,优选高度易消化的中链表面活性剂。此外,对于低渗透性药物,如尼罗替尼,易被肠道P-糖蛋白排出,表面活性剂的生物药学作用值得进一步考虑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号