当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alzheimer's disease risk modifier genes do not affect tau aggregate uptake, seeding or maintenance in cell models.
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1002/2211-5463.12928 Sourav Kolay 1 , Marc I Diamond 1
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1002/2211-5463.12928 Sourav Kolay 1 , Marc I Diamond 1
Affiliation
|
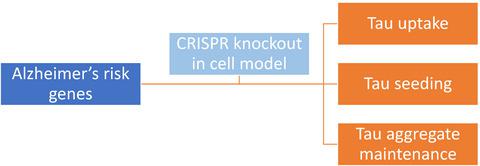
Alzheimer's disease (AD) afflicts millions of people worldwide and is caused by accumulated amyloid beta and tau pathology. Progression of tau pathology in AD may utilize prion mechanisms of propagation in which pathological tau aggregates released from one cell are taken up by neighboring or connected cells and act as templates for their own replication, a process termed ‘seeding’. We have used HEK293T cells to model various aspects of pathological tau propagation, including uptake of tau aggregates, induced seeding by exogenous aggregates, seeding caused by Lipofectamine‐mediated delivery to the cell interior, and stable maintenance of aggregates in dividing cells. The factors that regulate these processes are not well understood, and we hypothesized that AD risk modifier genes might play a role. We identified 22 genes strongly linked to AD via meta‐analysis of genome‐wide association study (GWAS). We used CRISPR/Cas9 to individually knock out each gene in HEK293T cells and verified disruption using genomic sequencing. We then tested the effect of gene knockout in tau aggregate uptake, naked and Lipofectamine‐mediated seeding, and aggregate maintenance in these cultured cell lines. GWAS gene knockouts had no effect in these models of tau pathology. With obvious caveats due to the model systems used, these results imply that the 22 AD risk modifier genes are unlikely to directly modulate tau uptake, seeding, or aggregate maintenance in a cell‐autonomous fashion.
中文翻译:

阿尔茨海默病风险修饰基因不影响细胞模型中的 tau 聚集体摄取、接种或维持。
阿尔茨海默病 (AD) 折磨着全世界数百万人,是由积累的淀粉样蛋白 β 和 tau 病理学引起的。AD 中 tau 病理的进展可能利用朊病毒传播机制,其中从一个细胞释放的病理性 tau 聚集体被相邻或连接的细胞吸收,并作为它们自身复制的模板,这一过程称为“播种”。我们使用 HEK293T 细胞对病理性 tau 传播的各个方面进行建模,包括 tau 聚集体的摄取、外源性聚集体的诱导接种、Lipofectamine 介导的细胞内部递送引起的接种以及分裂细胞中聚集体的稳定维持。调节这些过程的因素尚不清楚,我们假设 AD 风险修饰基因可能起作用。我们通过全基因组关联研究 (GWAS) 的荟萃分析确定了 22 个与 AD 密切相关的基因。我们使用 CRISPR/Cas9 单独敲除 HEK293T 细胞中的每个基因,并使用基因组测序验证破坏。然后,我们测试了基因敲除对 tau 聚集体吸收、裸露和 Lipofectamine 介导的接种以及这些培养细胞系中聚集体维持的影响。GWAS 基因敲除对这些 tau 病理模型没有影响。由于使用的模型系统有明显的警告,这些结果意味着 22 个 AD 风险修饰基因不太可能以细胞自主方式直接调节 tau 摄取、接种或聚集维持。然后,我们测试了基因敲除对 tau 聚集体摄取、裸露和 Lipofectamine 介导的接种以及这些培养细胞系中聚集体维持的影响。GWAS 基因敲除对这些 tau 病理模型没有影响。由于使用的模型系统有明显的警告,这些结果意味着 22 个 AD 风险修饰基因不太可能以细胞自主方式直接调节 tau 摄取、接种或聚集维持。然后,我们测试了基因敲除对 tau 聚集体摄取、裸露和 Lipofectamine 介导的接种以及这些培养细胞系中聚集体维持的影响。GWAS 基因敲除对这些 tau 病理模型没有影响。由于使用的模型系统有明显的警告,这些结果意味着 22 个 AD 风险修饰基因不太可能以细胞自主方式直接调节 tau 摄取、接种或聚集维持。
更新日期:2020-09-01
中文翻译:

阿尔茨海默病风险修饰基因不影响细胞模型中的 tau 聚集体摄取、接种或维持。
阿尔茨海默病 (AD) 折磨着全世界数百万人,是由积累的淀粉样蛋白 β 和 tau 病理学引起的。AD 中 tau 病理的进展可能利用朊病毒传播机制,其中从一个细胞释放的病理性 tau 聚集体被相邻或连接的细胞吸收,并作为它们自身复制的模板,这一过程称为“播种”。我们使用 HEK293T 细胞对病理性 tau 传播的各个方面进行建模,包括 tau 聚集体的摄取、外源性聚集体的诱导接种、Lipofectamine 介导的细胞内部递送引起的接种以及分裂细胞中聚集体的稳定维持。调节这些过程的因素尚不清楚,我们假设 AD 风险修饰基因可能起作用。我们通过全基因组关联研究 (GWAS) 的荟萃分析确定了 22 个与 AD 密切相关的基因。我们使用 CRISPR/Cas9 单独敲除 HEK293T 细胞中的每个基因,并使用基因组测序验证破坏。然后,我们测试了基因敲除对 tau 聚集体吸收、裸露和 Lipofectamine 介导的接种以及这些培养细胞系中聚集体维持的影响。GWAS 基因敲除对这些 tau 病理模型没有影响。由于使用的模型系统有明显的警告,这些结果意味着 22 个 AD 风险修饰基因不太可能以细胞自主方式直接调节 tau 摄取、接种或聚集维持。然后,我们测试了基因敲除对 tau 聚集体摄取、裸露和 Lipofectamine 介导的接种以及这些培养细胞系中聚集体维持的影响。GWAS 基因敲除对这些 tau 病理模型没有影响。由于使用的模型系统有明显的警告,这些结果意味着 22 个 AD 风险修饰基因不太可能以细胞自主方式直接调节 tau 摄取、接种或聚集维持。然后,我们测试了基因敲除对 tau 聚集体摄取、裸露和 Lipofectamine 介导的接种以及这些培养细胞系中聚集体维持的影响。GWAS 基因敲除对这些 tau 病理模型没有影响。由于使用的模型系统有明显的警告,这些结果意味着 22 个 AD 风险修饰基因不太可能以细胞自主方式直接调节 tau 摄取、接种或聚集维持。









































 京公网安备 11010802027423号
京公网安备 11010802027423号