Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hibernating ribosomes exhibit chaperoning activity but can resist unfolded protein‐mediated subunit dissociation
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-07-10 , DOI: 10.1111/febs.15479 Sehnaz Ferdosh 1 , Senjuti Banerjee 1 , Bani K Pathak 2 , Jayati Sengupta 2 , Chandana Barat 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-07-10 , DOI: 10.1111/febs.15479 Sehnaz Ferdosh 1 , Senjuti Banerjee 1 , Bani K Pathak 2 , Jayati Sengupta 2 , Chandana Barat 1
Affiliation
|
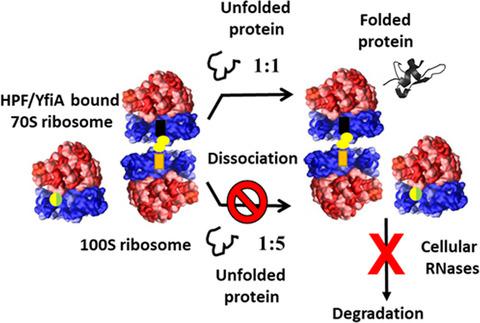
Ribosome hibernation is a prominent cellular strategy to modulate protein synthesis during starvation and the stationary phase of bacterial cell growth. Translational suppression involves the formation of either factor‐bound inactive 70S monomers or dimeric 100S hibernating ribosomal complexes, the biological significance of which is poorly understood. Here, we demonstrate that the Escherichia coli 70S ribosome associated with stationary phase factors hibernation promoting factor or protein Y or ribosome‐associated inhibitor A and the 100S ribosome isolated from both Gram‐negative and Gram‐positive bacteria are resistant to unfolded protein‐mediated subunit dissociation and subsequent degradation by cellular ribonucleases. Considering that the increase in cellular stress is accompanied by accumulation of unfolded proteins, such resistance of hibernating ribosomes towards dissociation might contribute to their maintenance during the stationary phase. Analysis of existing structures provided clues on the mechanism of inhibition of the unfolded protein‐mediated disassembly in case of hibernating factor‐bound ribosome. Further, the factor‐bound 70S and 100S ribosomes can suppress protein aggregation and assist in protein folding. The chaperoning activity of these ribosomes is the first evidence of a potential biological activity of the hibernating ribosome that might be crucial for cell survival under stress conditions.
中文翻译:

冬眠核糖体具有伴侣活性,但可以抵抗未折叠的蛋白质介导的亚基解离
核糖体冬眠是一种重要的细胞策略,可在饥饿和细菌细胞生长的稳定期调节蛋白合成。翻译抑制涉及形成因子结合的无活性70S单体或二聚体100S冬眠核糖体复合物的形成,人们对其生物学意义了解甚少。在这里,我们证明了大肠杆菌与稳定期因子冬眠促进因子或蛋白Y或核糖体相关抑制剂A相关的70S核糖体以及从革兰氏阴性和革兰氏阳性细菌中分离的100S核糖体对未折叠的蛋白质介导的亚基解离和随后的细胞核糖核酸降解具有抵抗力。考虑到细胞应激的增加伴随着未折叠蛋白质的积累,这种冬眠核糖体对解离的抵抗力可能有助于它们在固定期的维持。对现有结构的分析为在冬眠因子结合核糖体的情况下抑制未折叠的蛋白质介导的分解的机制提供了线索。此外,因子结合的70S和100S核糖体可以抑制蛋白质聚集并协助蛋白质折叠。
更新日期:2020-07-10
中文翻译:

冬眠核糖体具有伴侣活性,但可以抵抗未折叠的蛋白质介导的亚基解离
核糖体冬眠是一种重要的细胞策略,可在饥饿和细菌细胞生长的稳定期调节蛋白合成。翻译抑制涉及形成因子结合的无活性70S单体或二聚体100S冬眠核糖体复合物的形成,人们对其生物学意义了解甚少。在这里,我们证明了大肠杆菌与稳定期因子冬眠促进因子或蛋白Y或核糖体相关抑制剂A相关的70S核糖体以及从革兰氏阴性和革兰氏阳性细菌中分离的100S核糖体对未折叠的蛋白质介导的亚基解离和随后的细胞核糖核酸降解具有抵抗力。考虑到细胞应激的增加伴随着未折叠蛋白质的积累,这种冬眠核糖体对解离的抵抗力可能有助于它们在固定期的维持。对现有结构的分析为在冬眠因子结合核糖体的情况下抑制未折叠的蛋白质介导的分解的机制提供了线索。此外,因子结合的70S和100S核糖体可以抑制蛋白质聚集并协助蛋白质折叠。











































 京公网安备 11010802027423号
京公网安备 11010802027423号