当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
S6 N2 O15 -A Nitrogen-Poor Sulfur Nitride Oxide, and the Anhydride of Nitrido-tris-Sulfuric Acid.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-10 , DOI: 10.1002/anie.202005056 David van Gerven 1 , Mathias S Wickleder 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-10 , DOI: 10.1002/anie.202005056 David van Gerven 1 , Mathias S Wickleder 1
Affiliation
|
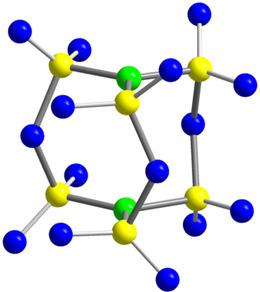
The reaction of hexachlorophosphazene, P3N3Cl6, with SO3 leads to the new sulfur nitride oxide S6N2O15. The compound displays an extraordinarily low nitrogen content and exhibits a bicyclic cage structure according to the formulation N{S(O)2O(O)2S}3N, with both nitrogen atoms in trigonal planar coordination of sulfur atoms. Interestingly, the new nitride oxide can be also seen as the anhydride of nitrido‐tris‐sulfuric acid, N(SO3H)3.
中文翻译:

S6 N2 O15 -一种贫氮硫氮氧化物,以及亚硝基三硫酸的酸酐。
六氯磷腈P 3 N 3 Cl 6与SO 3反应生成新的氮氧化硫S 6 N 2 O 15 。该化合物显示出极低的氮含量,并根据公式 N{S(O) 2 O(O) 2 S} 3 N 显示出双环笼式结构,其中两个氮原子均与硫原子呈三角平面配位。有趣的是,新的氮氧化物也可以被视为亚硝基三硫酸的酸酐,N(SO 3 H) 3 。
更新日期:2020-09-15
中文翻译:

S6 N2 O15 -一种贫氮硫氮氧化物,以及亚硝基三硫酸的酸酐。
六氯磷腈P 3 N 3 Cl 6与SO 3反应生成新的氮氧化硫S 6 N 2 O 15 。该化合物显示出极低的氮含量,并根据公式 N{S(O) 2 O(O) 2 S} 3 N 显示出双环笼式结构,其中两个氮原子均与硫原子呈三角平面配位。有趣的是,新的氮氧化物也可以被视为亚硝基三硫酸的酸酐,N(SO 3 H) 3 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号