当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atom Exchange Versus Reconstruction: (Gex As4-x )x- (x=2, 3) as Building Blocks for the Supertetrahedral Zintl Cluster [Au6 (Ge3 As)(Ge2 As2 )3 ]3.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-09 , DOI: 10.1002/anie.202008108 Fuxing Pan 1 , Lukas Guggolz 1 , Florian Weigend 1 , Stefanie Dehnen 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-09 , DOI: 10.1002/anie.202008108 Fuxing Pan 1 , Lukas Guggolz 1 , Florian Weigend 1 , Stefanie Dehnen 1
Affiliation
|
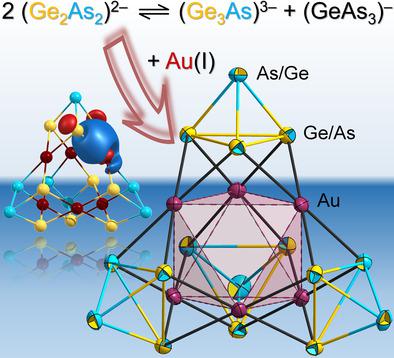
The Zintl anion (Ge2As2)2− represents an isostructural and isoelectronic binary counterpart of yellow arsenic, yet without being studied with the same intensity so far. Upon introducing [(PPh3)AuMe] into the 1,2‐diaminoethane (en) solution of (Ge2As2)2−, the heterometallic cluster anion [Au6(Ge3As)(Ge2As2)3]3− is obtained as its salt [K(crypt‐222)]3[Au6(Ge3As)(Ge2As2)3]⋅en⋅2 tol (1). The anion represents a rare example of a superpolyhedral Zintl cluster, and it comprises the largest number of Au atoms relative to main group (semi)metal atoms in such clusters. The overall supertetrahedral structure is based on a (non‐bonding) octahedron of six Au atoms that is face‐capped by four (GexAs4−x)x− (x=2, 3) units. The Au atoms bind to four main group atoms in a rectangular manner, and this way hold the four units together to form this unprecedented architecture. The presence of one (Ge3As)3− unit besides three (Ge2As2)2− units as a consequence of an exchange reaction in solution was verified by detailed quantum chemical (DFT) calculations, which ruled out all other compositions besides [Au6(Ge3As)(Ge2As2)3]3−. Reactions of the heavier homologues (Tt2Pn2)2− (Tt=Sn, Pb; Pn=Sb, Bi) did not yield clusters corresponding to that in 1, but dimers of ternary nine‐vertex clusters, {[AuTt5Pn3]2}4− (in 2–4; Tt/Pn=Sn/Sb, Sn/Bi, Pb/Sb), since the underlying pseudo‐tetrahedral units comprising heavier atoms do not tend to undergo the said exchange reactions as readily as (Ge2As2)2−, according to the DFT calculations.
中文翻译:

原子交换与重建:(Gex As4-x )x- (x=2, 3) 作为超四面体 Zintl 簇的构建块 [Au6 (Ge3 As)(Ge2 As2 )3 ]3。
Zintl 阴离子 (Ge 2 As 2 ) 2−代表黄砷的同构和等电子二元对应物,但迄今为止尚未进行相同强度的研究。将[(PPh 3 )AuMe]引入(Ge 2 As 2 ) 2−的1,2-二氨基乙烷(en)溶液中后,异金属簇阴离子[Au 6 (Ge 3 As)(Ge 2 As 2 ) 3 ] 3−以其盐形式获得 [K(crypt‐222)] 3 [Au 6 (Ge 3 As)(Ge 2 As 2 ) 3 ]⋅en⋅2 tol ( 1 )。该阴离子代表了超多面体 Zintl 簇的罕见例子,并且相对于此类簇中的主族(半)金属原子,它包含最多数量的 Au 原子。整体超四面体结构基于六个金原子的(非键合)八面体,其面由四个 (Ge x As 4− x ) x − ( x =2, 3) 单元覆盖。Au原子以矩形方式与四个主族原子结合,从而将四个单元结合在一起形成这种前所未有的结构。通过详细的量子化学 (DFT) 计算验证了溶液中交换反应导致的除三个 (Ge 2 As 2 ) 2− 单元外还存在一个( Ge 3 As) 3− 单元,这排除了除[Au 6 (Ge 3 As)(Ge 2 As 2 ) 3 ] 3−。较重的同系物 (Tt 2 Pn 2 ) 2− (Tt=Sn, Pb; Pn=Sb, Bi) 的反应没有产生与1中对应的簇,而是三元九顶点簇的二聚体,{[AuTt 5 Pn 3 ] 2 } 4−(在2 – 4中;Tt/Pn=Sn/Sb、Sn/Bi、Pb/Sb),因为包含较重原子的基础伪四面体单元不容易发生所述交换反应根据DFT计算为(Ge 2 As 2 ) 2− 。
更新日期:2020-09-08
中文翻译:

原子交换与重建:(Gex As4-x )x- (x=2, 3) 作为超四面体 Zintl 簇的构建块 [Au6 (Ge3 As)(Ge2 As2 )3 ]3。
Zintl 阴离子 (Ge 2 As 2 ) 2−代表黄砷的同构和等电子二元对应物,但迄今为止尚未进行相同强度的研究。将[(PPh 3 )AuMe]引入(Ge 2 As 2 ) 2−的1,2-二氨基乙烷(en)溶液中后,异金属簇阴离子[Au 6 (Ge 3 As)(Ge 2 As 2 ) 3 ] 3−以其盐形式获得 [K(crypt‐222)] 3 [Au 6 (Ge 3 As)(Ge 2 As 2 ) 3 ]⋅en⋅2 tol ( 1 )。该阴离子代表了超多面体 Zintl 簇的罕见例子,并且相对于此类簇中的主族(半)金属原子,它包含最多数量的 Au 原子。整体超四面体结构基于六个金原子的(非键合)八面体,其面由四个 (Ge x As 4− x ) x − ( x =2, 3) 单元覆盖。Au原子以矩形方式与四个主族原子结合,从而将四个单元结合在一起形成这种前所未有的结构。通过详细的量子化学 (DFT) 计算验证了溶液中交换反应导致的除三个 (Ge 2 As 2 ) 2− 单元外还存在一个( Ge 3 As) 3− 单元,这排除了除[Au 6 (Ge 3 As)(Ge 2 As 2 ) 3 ] 3−。较重的同系物 (Tt 2 Pn 2 ) 2− (Tt=Sn, Pb; Pn=Sb, Bi) 的反应没有产生与1中对应的簇,而是三元九顶点簇的二聚体,{[AuTt 5 Pn 3 ] 2 } 4−(在2 – 4中;Tt/Pn=Sn/Sb、Sn/Bi、Pb/Sb),因为包含较重原子的基础伪四面体单元不容易发生所述交换反应根据DFT计算为(Ge 2 As 2 ) 2− 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号