当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Density, Viscosity and Electrical Conductivity of Alcohol Solutions of 2,2-Diethyl-1,1,3,3-tetramethylguanidinium Bis(trifluoromethylsulfonyl)imide
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106241 Xiaoxing Lu , Shaoxia Xie , Jiayi Zhang , Qunfang Lei , Wenjun Fang
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106241 Xiaoxing Lu , Shaoxia Xie , Jiayi Zhang , Qunfang Lei , Wenjun Fang
|
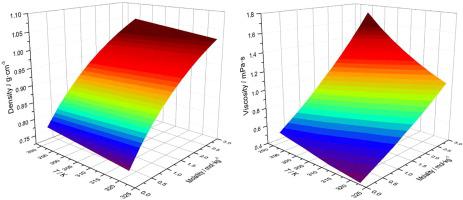
Abstract Density, viscosity and electrical conductivity are measured for the methanol and ethanol solutions of the ionic liquid (IL), 2,2-diethyl-1,1,3,3-tetramethylguanidinium bis(trifluoromethylsulfonyl)imide ([TMG(C2)2][NTf2]), at the molality up to 3 mol·kg−1, and the interactions are explored at the molecular level. The concentration dependence of the physicochemical properties is evaluated by empirical models including the Redlich–Kister, the Jones–Dole and the Casteel–Amis equations. The obtained interaction coefficients from viscosity measurements indicate the structure making effect in both systems, by which alcohol molecules gather around the ionic moieties through significant ion–dipole interactions. According to the calculated apparent molar volume and molar conductivity, ion aggregation occurs to form ion pairs when a specific concentration (c0) is reached. The IL behaves as a weak electrolyte in alcohols, and ethanol is more efficient to dissociate IL with a higher value of c0.
中文翻译:

2,2-二乙基-1,1,3,3-四甲基胍双(三氟甲基磺酰)亚胺醇溶液的密度、粘度和电导率
摘要 对离子液体 (IL), 2,2-二乙基-1,1,3,3-四甲基胍双(三氟甲基磺酰)亚胺 ([TMG(C2)2 ][NTf2]),在高达 3 mol·kg-1 的摩尔浓度下,并在分子水平上探索了相互作用。物理化学性质的浓度依赖性通过经验模型进行评估,包括 Redlich-Kister、Jones-Dole 和 Casteel-Amis 方程。从粘度测量中获得的相互作用系数表明两个系统中的结构形成效应,醇分子通过显着的离子-偶极相互作用聚集在离子部分周围。根据计算的表观摩尔体积和摩尔电导率,当达到特定浓度 (c0) 时,会发生离子聚集以形成离子对。IL 在醇类中表现为弱电解质,乙醇在 c0 值较高时更有效地解离 IL。
更新日期:2020-12-01
中文翻译:

2,2-二乙基-1,1,3,3-四甲基胍双(三氟甲基磺酰)亚胺醇溶液的密度、粘度和电导率
摘要 对离子液体 (IL), 2,2-二乙基-1,1,3,3-四甲基胍双(三氟甲基磺酰)亚胺 ([TMG(C2)2 ][NTf2]),在高达 3 mol·kg-1 的摩尔浓度下,并在分子水平上探索了相互作用。物理化学性质的浓度依赖性通过经验模型进行评估,包括 Redlich-Kister、Jones-Dole 和 Casteel-Amis 方程。从粘度测量中获得的相互作用系数表明两个系统中的结构形成效应,醇分子通过显着的离子-偶极相互作用聚集在离子部分周围。根据计算的表观摩尔体积和摩尔电导率,当达到特定浓度 (c0) 时,会发生离子聚集以形成离子对。IL 在醇类中表现为弱电解质,乙醇在 c0 值较高时更有效地解离 IL。











































 京公网安备 11010802027423号
京公网安备 11010802027423号