Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-07-10 , DOI: 10.1016/j.jsb.2020.107571 Abhiruchi Kant 1 , Airi Palva 2 , Ingemar von Ossowski 2 , Vengadesan Krishnan 3
|
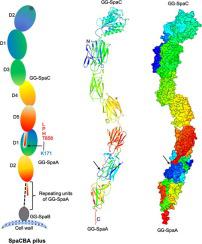
Adhesion to cell surfaces is an essential and early prerequisite for successful host colonization by bacteria, and in most instances involves the specificities of various adhesins. Among bacterial Gram-positives, some genera and species mediate attachment to host cells by using long non-flagellar appendages called sortase-dependent pili. A case in point is the beneficial Lactobacillus rhamnosus GG gut-adapted strain that produces the so-called SpaCBA pilus, a structure noted for its promiscuous binding to intestinal mucus and collagen. Structurally, SpaCBA pili are heteropolymers of three different pilin-protein subunits, each with its own location and function in the pilus: backbone SpaA for length, basal SpaB for anchoring, and tip SpaC for adhesion. Previously, we solved the SpaA tertiary structure by X-ray crystallography and also reported on the crystallization of SpaB and SpaC. Here, we reveal the full-length high-resolution (1.9 Å) crystal structure of SpaC, a first for a sortase-dependent pilus-bearing commensal. The SpaC structure, unlike the representative four-domain architecture of other Gram-positive tip pilins, espouses an atypically longer five-domain arrangement that includes N-terminal ‘binding’ and C-terminal ‘stalk’ regions of two and three domains, respectively. With the prospect of establishing new mechanistic insights, we provide a structural basis for the multi-substrate binding nature of SpaC, as well as a structural model that reconciles its exclusive localization at the SpaCBA pilus tip.
中文翻译:

乳杆菌 SpaC 的晶体结构揭示了一种非典型的五域菌毛尖端粘附素:在 SpaCBA 菌毛中暴露其底物结合和组装。
对细胞表面的粘附是细菌成功定殖宿主的必要和早期先决条件,并且在大多数情况下涉及各种粘附素的特异性。在革兰氏阳性菌中,一些属和种通过使用称为分选酶依赖性菌毛的长非鞭毛附属物介导对宿主细胞的附着。一个很好的例子是有益的鼠李糖乳杆菌GG 肠道适应菌株,产生所谓的 SpaCBA 菌毛,这种结构以其与肠道粘液和胶原蛋白的混杂结合而著称。在结构上,SpaCBA 菌毛是三种不同菌毛蛋白亚基的杂聚物,每个亚基在菌毛中都有自己的位置和功能:主链 SpaA 用于长度,基础 SpaB 用于锚定,尖端 SpaC 用于粘附。以前,我们通过 X 射线晶体学解析了 SpaA 三级结构,并报告了 SpaB 和 SpaC 的结晶。在这里,我们揭示了 SpaC 的全长高分辨率 (1.9 Å) 晶体结构,这是第一个依赖分选酶的带有菌毛的共生体。SpaC 结构,与其他革兰氏阳性提示菌毛蛋白的代表性四域架构不同,支持非典型较长的五域排列,分别包括两个和三个域的 N 端“结合”和 C 端“茎”区域。随着建立新机制见解的前景,我们为 SpaC 的多底物结合性质提供了结构基础,以及协调其在 SpaCBA 菌毛尖端的独家定位的结构模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号