当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic and structural characterization of whey protein aggregation in a millifluidic continuous process
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.foodhyd.2020.106137 Alice Vilotte , Hugues Bodiguel , Komla Ako , Deniz Z. Gunes , Christophe Schmitt , Clément de Loubens
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.foodhyd.2020.106137 Alice Vilotte , Hugues Bodiguel , Komla Ako , Deniz Z. Gunes , Christophe Schmitt , Clément de Loubens
|
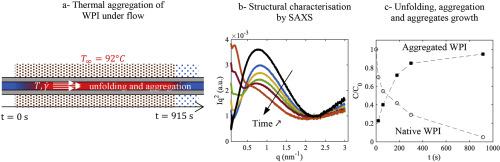
Abstract Whey protein isolates (WPI) can be aggregated upon heating to create new functional properties (e.g. texture), which depend on aggregate size and structural properties. In industrial conditions, aggregates are obtained in continuous processes at high temperature ( ≥ 75∘C) in few minutes. When studying the kinetics of WPI aggregation at high temperature and under flow, one major issue is to develop a process in which heat transfer does not limit aggregation. To this end, we used a down-scaling approach in which a WPI solution flows in a heated capillary tube. We show that this process makes it possible to study both the kinetics of aggregation after few seconds and its dependence with the mean shear rate in isothermal conditions. The size and mass of aggregates and protein conformation were characterized by small-angle X-ray scattering and resonant mass measurement for a single physico-chemical condition (pH 7.0, 10 mM NaCl, 92∘C, 4% w/w WPI) which led to sub-micrometric aggregates. Firstly, we report that the size of aggregates were three times larger than when produced in a test tube. Secondly, the size and mass of aggregates reached a steady-state value in a few seconds, whereas the kinetics of aggregation and denaturation had a characteristic time of few minutes. Thirdly, the shear rate had no significant effect on the size of the aggregates, or on the aggregation kinetics. We concluded that WPI aggregation at 92∘C is limited by a step of nucleation, and that the fact that aggregates produced in test tube were smaller is due to a slower thermalization.
中文翻译:

微流体连续过程中乳清蛋白聚集的动力学和结构表征
摘要 乳清蛋白分离物 (WPI) 可以在加热时聚集以产生新的功能特性(例如质地),这取决于聚集体的大小和结构特性。在工业条件下,在几分钟内在高温(≥75∘C)的连续过程中获得骨料。在研究高温和流动下 WPI 聚集的动力学时,一个主要问题是开发一种热传递不限制聚集的过程。为此,我们使用了一种按比例缩小的方法,其中 WPI 溶液在加热的毛细管中流动。我们表明,这个过程使得研究几秒钟后聚集的动力学及其与等温条件下平均剪切速率的关系成为可能。聚集体和蛋白质构象的大小和质量通过单一物理化学条件(pH 7.0、10 mM NaCl、92∘C、4% w/w WPI)的小角度X射线散射和共振质量测量表征导致亚微米聚集体。首先,我们报告聚集体的大小是在试管中生产时的三倍。其次,聚集体的大小和质量在几秒钟内达到稳态值,而聚集和变性的动力学具有几分钟的特征时间。第三,剪切速率对聚集体的大小或聚集动力学没有显着影响。我们得出结论,92∘C 下的 WPI 聚集受到成核步骤的限制,并且试管中产生的聚集体较小的事实是由于较慢的热化。
更新日期:2021-01-01
中文翻译:

微流体连续过程中乳清蛋白聚集的动力学和结构表征
摘要 乳清蛋白分离物 (WPI) 可以在加热时聚集以产生新的功能特性(例如质地),这取决于聚集体的大小和结构特性。在工业条件下,在几分钟内在高温(≥75∘C)的连续过程中获得骨料。在研究高温和流动下 WPI 聚集的动力学时,一个主要问题是开发一种热传递不限制聚集的过程。为此,我们使用了一种按比例缩小的方法,其中 WPI 溶液在加热的毛细管中流动。我们表明,这个过程使得研究几秒钟后聚集的动力学及其与等温条件下平均剪切速率的关系成为可能。聚集体和蛋白质构象的大小和质量通过单一物理化学条件(pH 7.0、10 mM NaCl、92∘C、4% w/w WPI)的小角度X射线散射和共振质量测量表征导致亚微米聚集体。首先,我们报告聚集体的大小是在试管中生产时的三倍。其次,聚集体的大小和质量在几秒钟内达到稳态值,而聚集和变性的动力学具有几分钟的特征时间。第三,剪切速率对聚集体的大小或聚集动力学没有显着影响。我们得出结论,92∘C 下的 WPI 聚集受到成核步骤的限制,并且试管中产生的聚集体较小的事实是由于较慢的热化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号