Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-07-10 , DOI: 10.1016/j.apcatb.2020.119328 Felix Donat , Christoph R. Müller
|
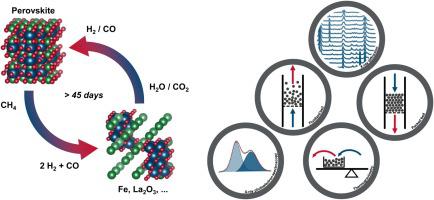
Chemical looping can provide attractive alternative process routes in which solid oxygen carriers function as lattice oxygen transfer agents, for example for the partial oxidation of methane. We report on the development of a perovskite-based oxygen carrier (La0.85Sr0.15Fe0.95Al0.05O3-δ) that enables the complete conversion of CH4 to a synthesis gas (a mixture of H2 and CO, selectivity > 99 %) through donation of its lattice oxygen (up to 9 wt%) at temperatures > 900 °C. The thermodynamic properties of the oxygen carrier permit its lattice oxygen to be replenished with CO2 or H2O, of which > 94 % is converted into CO or H2, respectively. The potential of this compositionally and structurally flexible oxygen carrier is demonstrated in a continuous experiment lasting more than 45 days (∼ 4050 redox cycles), in which all CH4 (reductant) and all CO2 (oxidant) is converted into a synthesis gas without CO2 contamination.
中文翻译:

使用化学环将CH 4无CO 2转化为合成气
化学环化可以提供有吸引力的替代工艺路线,其中固体氧载体充当晶格氧转移剂,例如用于甲烷的部分氧化。我们报道了钙钛矿基氧气载体(La 0.85 Sr 0.15 Fe 0.95 Al 0.05 O 3- δ)的发展,该载体能够将CH 4完全转化为合成气(H 2和CO的混合物,选择性> 99 %)通过在大于900°C的温度下提供其晶格氧(最高9 wt%)。氧载体的热力学性质允许其晶格氧被CO 2或H 2补充O,其中> 94%的O分别转化为CO或H 2。持续超过45天(〜4050氧化还原循环)的连续实验证明了这种具有结构和结构柔性的氧载体的潜力,其中所有CH 4(还原剂)和所有CO 2(氧化剂)被转化为合成气,而没有CO 2污染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号