当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The unique catalytic role of water in aromatic C-H activation at neutral pH: a combined NMR and DFT study of polyphenolic compounds.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0cp02826f Aneela Fayaz 1 , Michael G Siskos 2 , Panayiotis C Varras 2 , M Iqbal Choudhary 3 , Atia-Tul-Wahab 4 , Gerothanassis P Ioannis 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0cp02826f Aneela Fayaz 1 , Michael G Siskos 2 , Panayiotis C Varras 2 , M Iqbal Choudhary 3 , Atia-Tul-Wahab 4 , Gerothanassis P Ioannis 5
Affiliation
|
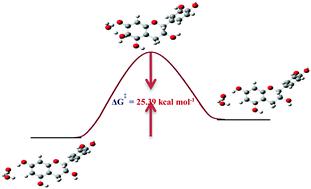
Direct activation of aromatic C–H bonds in polyphenolic compounds in a single step, without the use of late transition metals, is demonstrated with the use of D2O and common phosphate buffer at neutral pD and near ambient temperatures. Detailed variable temperature and pD 1H NMR studies were carried out to investigate, for the first time, the Gibbs activation energy (ΔG‡), the activation enthalpy (ΔH‡), and activation entropy (TΔS‡) of H/D exchange reactions of the natural product catechin and the model compounds resorcinol and phloroglucinol. NMR and DFT calculations support a catalytic cycle comprising two water molecules in a keto–enol tautomeric process. The reduction of ΔG‡ values due to the catalytic role of two molecules of water by a factor of 20–30 kcal mol−1 and the resulting acceleration of the H/D exchange rate by a factor of 1020–1030 should be compared with a minor reduction in ΔG‡ of 0.4 to 4.5 kcal mol−1 due to the effect of an additional electron donating oxygen group and the deprotonation of OH groups. It can therefore be concluded that although the H/D exchange process can be accelerated by a small amount of an acid or a base to break a C–H bond, water as a catalyst plays the major role. This approach opens a new vistas for the combined use of NMR and DFT studies as tools to understand the molecular basis of the catalytic role of water.
中文翻译:

在中性pH下,水在芳族CH活化中的独特催化作用:NMR和DFT结合研究多酚化合物。
通过在中性pD和接近环境温度下使用D 2 O和普通磷酸盐缓冲液,证明了在不使用后期过渡金属的情况下,一步即可直接激活多酚化合物中芳香族CH键的直接活化作用。详述可变温度和PD 1 1 H NMR研究进行了调查,对于第一次,吉布斯活化能(Δ ģ ‡),则活化焓(Δ ħ ‡),和活化熵(Ť Δ小号‡儿茶素与模型化合物间苯二酚和间苯三酚的H / D交换反应)。NMR和DFT计算支持在酮-烯醇互变异构过程中包含两个水分子的催化循环。Δ的还原ģ ‡值由于两个分子的水的催化作用由20-30千卡mol的因子-1通过10倍,将所得的H / d汇率的加速度20 -10 30应该是与之相比,ΔG ‡减小了0.4至4.5 kcal mol -1由于额外的给电子的氧基团和OH基团的去质子化的影响。因此可以得出结论,尽管可以通过少量的酸或碱破坏C–H键来加速H / D交换过程,但是水作为催化剂起着主要作用。这种方法为结合使用NMR和DFT研究作为了解水催化作用的分子基础的工具开辟了新的前景。
更新日期:2020-08-05
中文翻译:

在中性pH下,水在芳族CH活化中的独特催化作用:NMR和DFT结合研究多酚化合物。
通过在中性pD和接近环境温度下使用D 2 O和普通磷酸盐缓冲液,证明了在不使用后期过渡金属的情况下,一步即可直接激活多酚化合物中芳香族CH键的直接活化作用。详述可变温度和PD 1 1 H NMR研究进行了调查,对于第一次,吉布斯活化能(Δ ģ ‡),则活化焓(Δ ħ ‡),和活化熵(Ť Δ小号‡儿茶素与模型化合物间苯二酚和间苯三酚的H / D交换反应)。NMR和DFT计算支持在酮-烯醇互变异构过程中包含两个水分子的催化循环。Δ的还原ģ ‡值由于两个分子的水的催化作用由20-30千卡mol的因子-1通过10倍,将所得的H / d汇率的加速度20 -10 30应该是与之相比,ΔG ‡减小了0.4至4.5 kcal mol -1由于额外的给电子的氧基团和OH基团的去质子化的影响。因此可以得出结论,尽管可以通过少量的酸或碱破坏C–H键来加速H / D交换过程,但是水作为催化剂起着主要作用。这种方法为结合使用NMR和DFT研究作为了解水催化作用的分子基础的工具开辟了新的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号