当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interface catalysis by Pt nanocluster@Ni3N for bifunctional hydrogen evolution and oxygen evolution
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0qm00326c Yu Pei 1, 2, 3, 4, 5 , Babak Rezaei 3, 5, 6, 7 , Xuyang Zhang 3, 5, 6, 7 , Zichuang Li 1, 2, 3, 4, 5 , Hangjia Shen 3, 5, 6, 7 , Minghui Yang 3, 5, 6, 7, 8 , Jiacheng Wang 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2020-07-09 , DOI: 10.1039/d0qm00326c Yu Pei 1, 2, 3, 4, 5 , Babak Rezaei 3, 5, 6, 7 , Xuyang Zhang 3, 5, 6, 7 , Zichuang Li 1, 2, 3, 4, 5 , Hangjia Shen 3, 5, 6, 7 , Minghui Yang 3, 5, 6, 7, 8 , Jiacheng Wang 1, 2, 3, 4, 5
Affiliation
|
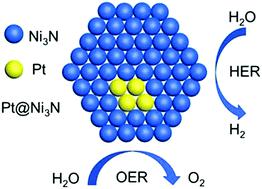
Due to their importance for practical applications in energy conversion, resolving the kinetic issues of water-dissociation in alkaline media for Pt-based electrocatalysts remains an ongoing challenge. Herein, the electronic properties of Pt could be modified with a nickel nitride (Ni3N) support through forming an interface of a low ratio of Pt nanoclusters (Pt NCs) via a simple impregnation approach combined with nitridation. 1.5Pt@Ni3N-360 can offer excellent bifunctional performance for both the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) which is competitive with commercial IrO2 and Pt/C catalysts under alkaline media. It shows a low overpotential at 10 mA cm−2 (285 and 71 mV) and a small Tafel slope (57 and 48 mV dec−1) for OER and HER, respectively. The excellent bifunctional electrochemical activity of 1.5Pt@Ni3N-360 is due to the interface and synergetic effect between Ni3N and dispersed Pt clusters, which changes the electron distribution of the material, so that the hydrogen adsorption energy tends to the optimal value (ΔGH* ∼ 0). It is believed that the utilization of low-ratio Pt as an co-electrocatalyst with a variety of metal nitrides opens new horizons and opportunities to fabricate more efficient electrocatalysts for broad applications in energy-related devices.
中文翻译:

Pt纳米簇@ Ni3N的界面催化双功能析氢和析氧
由于它们对于能量转换中的实际应用很重要,因此解决基于Pt的电催化剂在碱性介质中水离解的动力学问题仍然是一个持续的挑战。在本文中,可以通过简单的浸渍方法与氮化相结合形成低比例的Pt纳米团簇(Pt NCs)的界面,用氮化镍(Ni 3 N)载体修饰Pt的电子性质。1.5Pt@Ni 3 N-360可为氧气释放反应(OER)和氢气释放反应(HER)提供出色的双功能性能,在碱性介质下可与商用IrO 2和Pt / C催化剂竞争。在10 mA cm -2时显示出低过电位对于OER和HER分别为(285和71 mV)和小的Tafel斜率(57和48 mV dec -1)。1.5Pt@Ni 3 N-360的优异的双功能电化学活性归因于Ni 3 N与分散的Pt团簇之间的界面和协同作用,这改变了材料的电子分布,因此氢吸附能趋于最佳值(ΔG H *〜0)。人们相信,将低比率的Pt与多种金属氮化物作为助电催化剂的利用为制造更有效的电催化剂开辟了新的视野,并为在能源相关设备中的广泛应用提供了机会。
更新日期:2020-08-27
中文翻译:

Pt纳米簇@ Ni3N的界面催化双功能析氢和析氧
由于它们对于能量转换中的实际应用很重要,因此解决基于Pt的电催化剂在碱性介质中水离解的动力学问题仍然是一个持续的挑战。在本文中,可以通过简单的浸渍方法与氮化相结合形成低比例的Pt纳米团簇(Pt NCs)的界面,用氮化镍(Ni 3 N)载体修饰Pt的电子性质。1.5Pt@Ni 3 N-360可为氧气释放反应(OER)和氢气释放反应(HER)提供出色的双功能性能,在碱性介质下可与商用IrO 2和Pt / C催化剂竞争。在10 mA cm -2时显示出低过电位对于OER和HER分别为(285和71 mV)和小的Tafel斜率(57和48 mV dec -1)。1.5Pt@Ni 3 N-360的优异的双功能电化学活性归因于Ni 3 N与分散的Pt团簇之间的界面和协同作用,这改变了材料的电子分布,因此氢吸附能趋于最佳值(ΔG H *〜0)。人们相信,将低比率的Pt与多种金属氮化物作为助电催化剂的利用为制造更有效的电催化剂开辟了新的视野,并为在能源相关设备中的广泛应用提供了机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号