当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water-Gated Proton Transfer Dynamics in Respiratory Complex I
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-09 , DOI: 10.1021/jacs.0c02789 Max E Mühlbauer 1, 2 , Patricia Saura 1, 2 , Franziska Nuber 3 , Andrea Di Luca 1, 2 , Thorsten Friedrich 3 , Ville R I Kaila 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-09 , DOI: 10.1021/jacs.0c02789 Max E Mühlbauer 1, 2 , Patricia Saura 1, 2 , Franziska Nuber 3 , Andrea Di Luca 1, 2 , Thorsten Friedrich 3 , Ville R I Kaila 1, 2
Affiliation
|
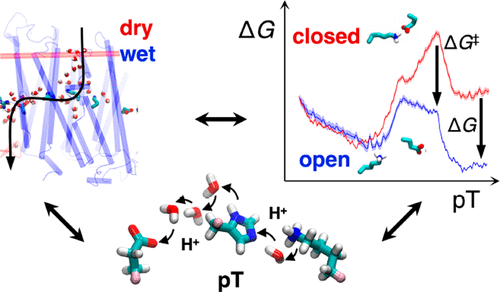
The respiratory complex I transduces redox energy into an electrochemical proton gradient in aerobic respiratory chains, powering energy-requiring processes in the cell. However, despite recently resolved molecular structures, the mechanism of this gigantic enzyme remains poorly understood. By combining large-scale quantum and classical simulations with site-directed mutagenesis and biophysical experiments, we show here how the conformational state of buried ion-pairs and water molecules control the protonation dynamics in the membrane domain of complex I and establish evolutionary conserved long-range coupling elements. We suggest that an electrostatic wave propagates in forward and reverse directions across the 200 Å long membrane domain during enzyme turnover, without significant dissipation of energy. Our findings demonstrate molecular principles that enable efficient long-range proton–electron coupling (PCET) and how perturbation of this PCET machinery may lead to development of mitochondrial disease.
中文翻译:

呼吸复合体 I 中的水门控质子转移动力学
呼吸复合物 I 将氧化还原能量转化为有氧呼吸链中的电化学质子梯度,为细胞中需要能量的过程提供动力。然而,尽管最近解析了分子结构,但对这种巨大酶的机制仍然知之甚少。通过将大规模量子和经典模拟与定点诱变和生物物理实验相结合,我们在这里展示了埋藏离子对和水分子的构象状态如何控制复合物 I 膜域中的质子化动力学,并建立进化保守的长-范围耦合元件。我们建议静电波在酶周转期间在 200 Å 长的膜域上正向和反向传播,而不会显着耗散能量。
更新日期:2020-07-09
中文翻译:

呼吸复合体 I 中的水门控质子转移动力学
呼吸复合物 I 将氧化还原能量转化为有氧呼吸链中的电化学质子梯度,为细胞中需要能量的过程提供动力。然而,尽管最近解析了分子结构,但对这种巨大酶的机制仍然知之甚少。通过将大规模量子和经典模拟与定点诱变和生物物理实验相结合,我们在这里展示了埋藏离子对和水分子的构象状态如何控制复合物 I 膜域中的质子化动力学,并建立进化保守的长-范围耦合元件。我们建议静电波在酶周转期间在 200 Å 长的膜域上正向和反向传播,而不会显着耗散能量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号