当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structure and C−H Bond Cleaving Reactivity of a Mononuclear CoIV-dinitrate Complex
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-08 , DOI: 10.1021/jacs.0c04368 Yubin M Kwon 1 , Yuri Lee 2 , Garrett E Evenson 1 , Timothy A Jackson 2 , Dong Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-08 , DOI: 10.1021/jacs.0c04368 Yubin M Kwon 1 , Yuri Lee 2 , Garrett E Evenson 1 , Timothy A Jackson 2 , Dong Wang 1
Affiliation
|
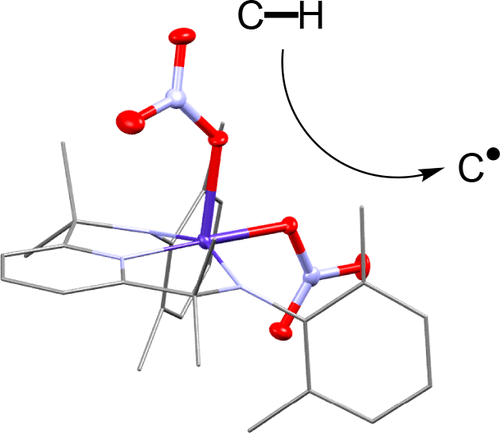
High-valent FeIV=O intermediates with a terminal metal-oxo moiety are key oxidants in many enzymatic and synthetic C-H bond oxidation reactions. While generating stable metal-oxo species for late transition metals remains synthetically challenging, notably, a number of high-valent non-oxo-metal species of late transition metals have been recently described as strong oxidants that activate C-H bonds. In this work, we obtained an unprecedented mononuclear CoIV-dinitrate complex (2) upon one-electron oxidation of its Co(III) precursor supported by a tridentate di-anionic N3 ligand. 2 was structurally characterized by X-ray crystallography, showing a square pyramidal geometry with two coordinated nitrate anions. Furthermore, characterization of 2 us-ing combined spectroscopic and computational methods revealed that 2 is a low-spin (S = 1/2) Co(IV) species with the unpaired electron located on the cobalt dz2 orbital, which is well positioned for substrate oxidations. Indeed, while having a high thermal stability, 2 is able to cleave sp3 C-H bonds up to 87 kcal/mol to afford rate constants and kinetic isotope effects (KIEs) of 2-6 that are comparable to other high-valent metal oxidants. The ability to oxidize strong C-H bonds has yet to be observed for CoIV-O and CoIII=O species previously reported. Therefore, 2 represents the first high-valent Co(IV) species that is both structurally character-ized by X-ray crystallography and is capable of activating strong C-H bonds.
中文翻译:

单核 CoIV-二硝酸酯配合物的晶体结构和 CH 键裂解反应性
具有末端金属氧部分的高价 FeIV=O 中间体是许多酶促和合成 CH 键氧化反应中的关键氧化剂。虽然为后过渡金属生成稳定的金属-氧代物质在合成上仍然具有挑战性,但值得注意的是,后过渡金属的许多高价非氧代金属物质最近被描述为激活CH键的强氧化剂。在这项工作中,我们通过三齿双阴离子 N3 配体支持的 Co(III) 前体的单电子氧化,获得了前所未有的单核 CoIV-二硝酸盐配合物 (2)。 2通过X射线晶体学进行了结构表征,显示出具有两个配位的硝酸根阴离子的方锥体几何形状。此外,使用组合光谱和计算方法对 2 进行表征表明,2 是一种低自旋 (S = 1/2) Co(IV) 物质,其不成对电子位于钴 dz2 轨道上,该轨道非常适合底物氧化。事实上,在具有高热稳定性的同时,2 能够以高达 87 kcal/mol 的能量裂解 sp3 CH 键,从而提供与其他高价金属氧化剂相当的 2-6 的速率常数和动力学同位素效应 (KIE)。此前报道的 CoIV-O 和 CoIII=O 物种尚未观察到氧化强 CH 键的能力。因此,2 代表第一个高价 Co(IV) 物种,其结构通过 X 射线晶体学进行表征,并且能够激活强 CH 键。
更新日期:2020-07-08
中文翻译:

单核 CoIV-二硝酸酯配合物的晶体结构和 CH 键裂解反应性
具有末端金属氧部分的高价 FeIV=O 中间体是许多酶促和合成 CH 键氧化反应中的关键氧化剂。虽然为后过渡金属生成稳定的金属-氧代物质在合成上仍然具有挑战性,但值得注意的是,后过渡金属的许多高价非氧代金属物质最近被描述为激活CH键的强氧化剂。在这项工作中,我们通过三齿双阴离子 N3 配体支持的 Co(III) 前体的单电子氧化,获得了前所未有的单核 CoIV-二硝酸盐配合物 (2)。 2通过X射线晶体学进行了结构表征,显示出具有两个配位的硝酸根阴离子的方锥体几何形状。此外,使用组合光谱和计算方法对 2 进行表征表明,2 是一种低自旋 (S = 1/2) Co(IV) 物质,其不成对电子位于钴 dz2 轨道上,该轨道非常适合底物氧化。事实上,在具有高热稳定性的同时,2 能够以高达 87 kcal/mol 的能量裂解 sp3 CH 键,从而提供与其他高价金属氧化剂相当的 2-6 的速率常数和动力学同位素效应 (KIE)。此前报道的 CoIV-O 和 CoIII=O 物种尚未观察到氧化强 CH 键的能力。因此,2 代表第一个高价 Co(IV) 物种,其结构通过 X 射线晶体学进行表征,并且能够激活强 CH 键。









































 京公网安备 11010802027423号
京公网安备 11010802027423号