当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Evaluation of the Stability and Capacity of r‐GO‐Wrapped Copper Antimony Chalcogenide Anode for Li‐Ion battery
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-07-09 , DOI: 10.1002/celc.202000625 Poonam Yadav 1, 2, 3 , Neha Sharma 4 , Apurva Patrike 1, 2 , Ylias M. Sabri 3 , Lathe A. Jones 3 , Manjusha V. Shelke 1, 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-07-09 , DOI: 10.1002/celc.202000625 Poonam Yadav 1, 2, 3 , Neha Sharma 4 , Apurva Patrike 1, 2 , Ylias M. Sabri 3 , Lathe A. Jones 3 , Manjusha V. Shelke 1, 2
Affiliation
|
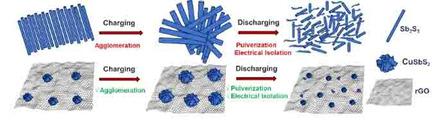
Poor cycling stability and capacity fade are primary concerns for next‐generation anode materials for Li‐ion batteries. In non‐carbonaceous anode materials, alloying with Li leads to volume increase that affects practical applications, and increase in particle size, amorphization and reduced conductivity can all lead to a loss of performance. In this work, binary antimony sulfide (Sb2S3) and ternary copper antimony sulfide (CuSbS2) are synthesized by a convenient solvothermal process. These materials are used to study the Li‐active/inactive concept, by incorporating Cu into Sb2S3 forming CuSbS2 wherein Cu is Li inactive whereas Sb is Li active. By direct comparison, we have shown that incorporating Cu into binary antimony sulfide (Sb2S3) resulting into ternary copper antimony sulfide (CuSbS2) addresses the problem of poor conductivity and capacity loss, as Cu provides conductivity leading to enhanced charge transfer and prevents Sb particle aggregation while charge‐discharge by exhibiting spectator or diluent ion effect. The better performance of CuSbS2 is associated with the better Li+ ion diffusion in the CuSbS2 (D =8.97×10−15 cm2 s−1) compared to Sb2S3 (D =2.76×10−15 cm2 s−1) and lower series resistance of CuSbS2 (R =4.70×105 Ω) compared to Sb2S3 (R =5.81×108 Ω). We have also investigated the composite with the addition of rGO. The CuSbS2‐rGO delivered a reversible capacity of 672 mAh g−1 after 1000 cycles at 200 mA g−1 which has shown best performance.
中文翻译:

锂离子电池r-GO包裹的铜锑硫族化物阳极稳定性和容量的电化学评估
循环稳定性差和容量衰减是锂离子电池下一代负极材料的主要问题。在非碳质阳极材料中,与Li合金化会导致体积增大,从而影响实际应用,而粒径增大,非晶化和电导率降低都可能导致性能下降。在这项工作中,通过方便的溶剂热法合成了二元硫化锑(Sb 2 S 3)和三元硫化铜锑(CuSbS 2)。通过将Cu掺入Sb 2 S 3中形成CuSbS 2,这些材料用于研究Li-活性/非活性概念。其中Cu是惰性的Li,而Sb是活性的Li。通过直接比较,我们发现将Cu掺入二元硫化锑(Sb 2 S 3)中,生成三元硫化铜锑(CuSbS 2)解决了电导率和容量损失较弱的问题,因为Cu提供了电导率,导致电荷转移增强,并且通过表现出旁观者或稀释剂离子效应,防止Sb粒子在放电时聚集。与Sb 2相比,CuSbS 2的性能更好与Li +离子在CuSbS 2中的扩散更好(D = 8.97×10 -15 cm 2 s -1)有关。小号3(d = 2.76×10 -15 厘米2个 小号-1)和CuSbS的较低的串联电阻2([R = 4.70×10 5 Ω)相比的Sb 2小号3([R = 5.81×10 8 Ω)。我们还研究了添加rGO的复合材料。CuSbS 2 -rGO在200 mA g -1下经过1000次循环后可提供672 mAh g -1的可逆容量,表现出最佳性能。
更新日期:2020-08-03
中文翻译:

锂离子电池r-GO包裹的铜锑硫族化物阳极稳定性和容量的电化学评估
循环稳定性差和容量衰减是锂离子电池下一代负极材料的主要问题。在非碳质阳极材料中,与Li合金化会导致体积增大,从而影响实际应用,而粒径增大,非晶化和电导率降低都可能导致性能下降。在这项工作中,通过方便的溶剂热法合成了二元硫化锑(Sb 2 S 3)和三元硫化铜锑(CuSbS 2)。通过将Cu掺入Sb 2 S 3中形成CuSbS 2,这些材料用于研究Li-活性/非活性概念。其中Cu是惰性的Li,而Sb是活性的Li。通过直接比较,我们发现将Cu掺入二元硫化锑(Sb 2 S 3)中,生成三元硫化铜锑(CuSbS 2)解决了电导率和容量损失较弱的问题,因为Cu提供了电导率,导致电荷转移增强,并且通过表现出旁观者或稀释剂离子效应,防止Sb粒子在放电时聚集。与Sb 2相比,CuSbS 2的性能更好与Li +离子在CuSbS 2中的扩散更好(D = 8.97×10 -15 cm 2 s -1)有关。小号3(d = 2.76×10 -15 厘米2个 小号-1)和CuSbS的较低的串联电阻2([R = 4.70×10 5 Ω)相比的Sb 2小号3([R = 5.81×10 8 Ω)。我们还研究了添加rGO的复合材料。CuSbS 2 -rGO在200 mA g -1下经过1000次循环后可提供672 mAh g -1的可逆容量,表现出最佳性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号