当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single‐molecule tracking reveals that the nucleoid‐associated protein HU plays a dual role in maintaining proper nucleoid volume through differential interactions with chromosomal DNA
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-07-08 , DOI: 10.1111/mmi.14572 Kelsey Bettridge 1 , Subhash Verma 2 , Xiaoli Weng 2 , Sankar Adhya 2 , Jie Xiao 1
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-07-08 , DOI: 10.1111/mmi.14572 Kelsey Bettridge 1 , Subhash Verma 2 , Xiaoli Weng 2 , Sankar Adhya 2 , Jie Xiao 1
Affiliation
|
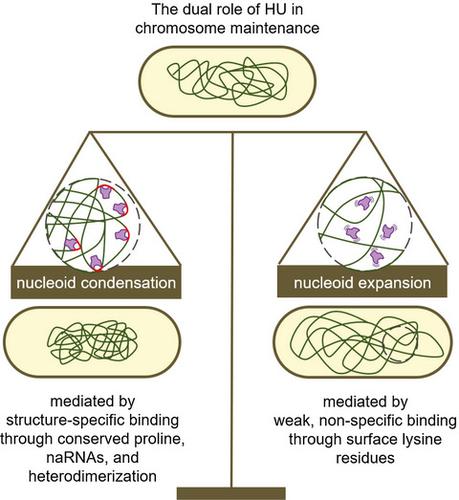
HU (Histone‐like protein from Escherichia coli strain U93) is the most conserved nucleoid‐associated protein in eubacteria, but how it impacts global chromosome organization is poorly understood. Using single‐molecule tracking, we demonstrate that HU exhibits nonspecific, weak, and transitory interactions with the chromosomal DNA. These interactions are largely mediated by three conserved, surface‐exposed lysine residues (triK), which were previously shown to be responsible for nonspecific binding to DNA. The loss of these weak, transitory interactions in a HUα(triKA) mutant results in an over‐condensed and mis‐segregated nucleoid. Mutating a conserved proline residue (P63A) in the HUα subunit, deleting the HUβ subunit, or deleting nucleoid‐associated naRNAs, each previously implicated in HU’s high‐affinity binding to kinked or cruciform DNA, leads to less dramatically altered interacting dynamics of HU compared to the HUα(triKA) mutant, but highly expanded nucleoids. Our results suggest HU plays a dual role in maintaining proper nucleoid volume through its differential interactions with chromosomal DNA. On the one hand, HU compacts the nucleoid through specific DNA structure‐binding interactions. On the other hand, it decondenses the nucleoid through many nonspecific, weak, and transitory interactions with the bulk chromosome. Such dynamic interactions may contribute to the viscoelastic properties and fluidity of the bacterial nucleoid to facilitate proper chromosome functions.
中文翻译:

单分子追踪表明,类核蛋白 HU 通过与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥双重作用
HU(来自大肠杆菌的组蛋白样蛋白)菌株 U93) 是真细菌中最保守的类核蛋白,但它如何影响全球染色体组织却知之甚少。使用单分子追踪,我们证明 HU 与染色体 DNA 表现出非特异性、弱和短暂的相互作用。这些相互作用主要由三个保守的、暴露于表面的赖氨酸残基 (triK) 介导,之前已证明这些残基与 DNA 的非特异性结合有关。HUα(triKA) 突变体中这些弱的、短暂的相互作用的丧失导致过度浓缩和错误分离的类核。突变 HUα 亚基中保守的脯氨酸残基 (P63A),删除 HUβ 亚基,或删除类核相关的 naRNA,每个以前都涉及 HU 与扭结或十字形 DNA 的高亲和力结合,与 HUα(triKA) 突变体相比,导致 HU 相互作用动力学的改变较小,但核仁高度扩展。我们的结果表明 HU 通过其与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥着双重作用。一方面,HU 通过特定的 DNA 结构结合相互作用来压缩类核。另一方面,它通过与大量染色体的许多非特异性、弱和短暂的相互作用来解压缩类核。这种动态相互作用可能有助于细菌类核的粘弹性和流动性,以促进适当的染色体功能。我们的结果表明 HU 通过其与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥着双重作用。一方面,HU 通过特定的 DNA 结构结合相互作用来压缩类核。另一方面,它通过与大量染色体的许多非特异性、弱和短暂的相互作用来解压缩类核。这种动态相互作用可能有助于细菌类核的粘弹性和流动性,以促进适当的染色体功能。我们的结果表明 HU 通过其与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥着双重作用。一方面,HU 通过特定的 DNA 结构结合相互作用来压缩类核。另一方面,它通过与大量染色体的许多非特异性、弱和短暂的相互作用来解压缩类核。这种动态相互作用可能有助于细菌类核的粘弹性和流动性,以促进适当的染色体功能。
更新日期:2020-07-08
中文翻译:

单分子追踪表明,类核蛋白 HU 通过与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥双重作用
HU(来自大肠杆菌的组蛋白样蛋白)菌株 U93) 是真细菌中最保守的类核蛋白,但它如何影响全球染色体组织却知之甚少。使用单分子追踪,我们证明 HU 与染色体 DNA 表现出非特异性、弱和短暂的相互作用。这些相互作用主要由三个保守的、暴露于表面的赖氨酸残基 (triK) 介导,之前已证明这些残基与 DNA 的非特异性结合有关。HUα(triKA) 突变体中这些弱的、短暂的相互作用的丧失导致过度浓缩和错误分离的类核。突变 HUα 亚基中保守的脯氨酸残基 (P63A),删除 HUβ 亚基,或删除类核相关的 naRNA,每个以前都涉及 HU 与扭结或十字形 DNA 的高亲和力结合,与 HUα(triKA) 突变体相比,导致 HU 相互作用动力学的改变较小,但核仁高度扩展。我们的结果表明 HU 通过其与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥着双重作用。一方面,HU 通过特定的 DNA 结构结合相互作用来压缩类核。另一方面,它通过与大量染色体的许多非特异性、弱和短暂的相互作用来解压缩类核。这种动态相互作用可能有助于细菌类核的粘弹性和流动性,以促进适当的染色体功能。我们的结果表明 HU 通过其与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥着双重作用。一方面,HU 通过特定的 DNA 结构结合相互作用来压缩类核。另一方面,它通过与大量染色体的许多非特异性、弱和短暂的相互作用来解压缩类核。这种动态相互作用可能有助于细菌类核的粘弹性和流动性,以促进适当的染色体功能。我们的结果表明 HU 通过其与染色体 DNA 的不同相互作用,在维持适当的类核体积方面发挥着双重作用。一方面,HU 通过特定的 DNA 结构结合相互作用来压缩类核。另一方面,它通过与大量染色体的许多非特异性、弱和短暂的相互作用来解压缩类核。这种动态相互作用可能有助于细菌类核的粘弹性和流动性,以促进适当的染色体功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号