当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of alkali metal cations on length and strength of hydrogen bonds in DNA base pairs.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-09 , DOI: 10.1002/cphc.202000434 Olga A Stasyuk 1 , Miquel Solà 1 , Marcel Swart 1, 2 , Célia Fonseca Guerra 3, 4 , Tadeusz Marek Krygowski 5 , Halina Szatylowicz 6
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-09 , DOI: 10.1002/cphc.202000434 Olga A Stasyuk 1 , Miquel Solà 1 , Marcel Swart 1, 2 , Célia Fonseca Guerra 3, 4 , Tadeusz Marek Krygowski 5 , Halina Szatylowicz 6
Affiliation
|
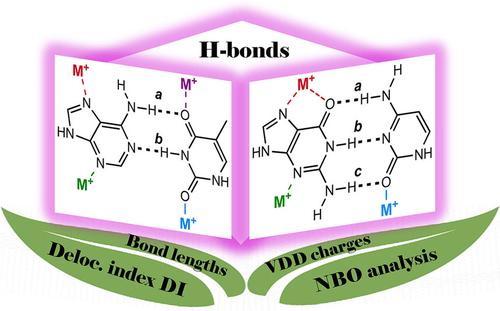
For many years, non‐covalently bonded complexes of nucleobases have attracted considerable interest. However, there is a lack of information about the nature of hydrogen bonding between nucleobases when the bonding is affected by metal coordination to one of the nucleobases, and how the individual hydrogen bonds and aromaticity of nucleobases respond to the presence of the metal cation. Here we report a DFT computational study of nucleobase pairs interacting with alkali metal cations. The metal cations contribute to the stabilization of the base pairs to varying degrees depending on their position. The energy decomposition analysis revealed that the nature of bonding between nucleobases does not change much upon metal coordination. The effect of the cations on individual hydrogen bonds were described by changes in VDD charges on frontier atoms, H‐bond length, bond energy from NBO analysis, and the delocalization index from QTAIM calculations. The aromaticity changes were determined by a HOMA index.
中文翻译:

碱金属阳离子对DNA碱基对中氢键的长度和强度的影响。
多年来,核苷的非共价键复合物引起了人们的极大兴趣。但是,当键受到与一个核碱基的金属配位影响时,以及与核碱基之间的氢键和芳香性如何响应金属阳离子的反应时,缺乏有关核碱基之间氢键性质的信息。在这里,我们报告与碱金属阳离子相互作用的核碱基对的DFT计算研究。金属阳离子根据其位置在不同程度上有助于碱基对的稳定化。能量分解分析表明,核碱基之间键合的性质在金属配位后不会发生太大变化。阳离子对单个氢键的影响通过前沿原子上VDD电荷的变化来描述,H键长度,NBO分析中的键能以及QTAIM计算中的离域指数。芳香度变化由HOMA指数确定。
更新日期:2020-09-15
中文翻译:

碱金属阳离子对DNA碱基对中氢键的长度和强度的影响。
多年来,核苷的非共价键复合物引起了人们的极大兴趣。但是,当键受到与一个核碱基的金属配位影响时,以及与核碱基之间的氢键和芳香性如何响应金属阳离子的反应时,缺乏有关核碱基之间氢键性质的信息。在这里,我们报告与碱金属阳离子相互作用的核碱基对的DFT计算研究。金属阳离子根据其位置在不同程度上有助于碱基对的稳定化。能量分解分析表明,核碱基之间键合的性质在金属配位后不会发生太大变化。阳离子对单个氢键的影响通过前沿原子上VDD电荷的变化来描述,H键长度,NBO分析中的键能以及QTAIM计算中的离域指数。芳香度变化由HOMA指数确定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号