当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the Enigma of Nonoxidative Conversion of Methane on Iron Single-Atom Catalysts.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-09 , DOI: 10.1002/anie.202003908 Yuan Liu 1, 2 , Jin-Cheng Liu 1 , Teng-Hao Li 3 , Zeng-Hui Duan 1 , Tian-Yu Zhang 4 , Ming Yan 3 , Wan-Lu Li 1 , Hai Xiao 1 , Yang-Gang Wang 2 , Chun-Ran Chang 3 , Jun Li 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-09 , DOI: 10.1002/anie.202003908 Yuan Liu 1, 2 , Jin-Cheng Liu 1 , Teng-Hao Li 3 , Zeng-Hui Duan 1 , Tian-Yu Zhang 4 , Ming Yan 3 , Wan-Lu Li 1 , Hai Xiao 1 , Yang-Gang Wang 2 , Chun-Ran Chang 3 , Jun Li 1, 2
Affiliation
|
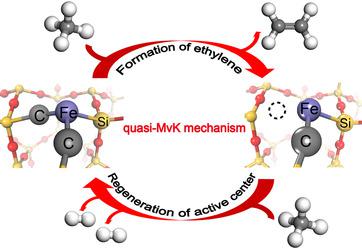
The direct, nonoxidative conversion of methane on a silica‐confined single‐atom iron catalyst is a landmark discovery in catalysis, but the proposed gas‐phase reaction mechanism is still open to discussion. Here, we report a surface reaction mechanism by computational modeling and simulations. The activation of methane occurs at the single iron site, whereas the dissociated methyl disfavors desorption into gas phase under the reactive conditions. In contrast, the dissociated methyl prefers transferring to adjacent carbon sites of the active center (Fe1©SiC2), followed by C−C coupling and hydrogen transfer to produce the main product (ethylene) via a key −CH−CH2 intermediate. We find a quasi Mars–van Krevelen (quasi‐MvK) surface reaction mechanism involving extracting and refilling the surface carbon atoms for the nonoxidative conversion of methane on Fe1©SiO2 and this surface process is identified to be more plausible than the alternative gas‐phase reaction mechanism.
中文翻译:

解开铁单原子催化剂上甲烷的非氧化转化之谜。
在二氧化硅限制的单原子铁催化剂上甲烷的直接,非氧化转化是催化领域的一个里程碑式的发现,但是所提出的气相反应机理仍需讨论。在这里,我们通过计算建模和仿真报告了一种表面反应机理。甲烷的活化发生在单个铁位点,而在反应条件下,离解的甲基不利于解吸成气相。相比之下,离解的甲基更喜欢转移到活性中心的相邻碳位点(Fe 1 ©SiC 2),然后进行C-C偶联和氢转移,从而通过键-CH-CH 2生成主要产物(乙烯)。中间。我们发现了一种准Mars-van Krevelen(准MvK)表面反应机制,涉及提取和重新填充表面碳原子以实现甲烷在Fe 1 ©SiO 2上的非氧化转化,并且该表面过程被认为比其他气体更可信阶段反应机理。
更新日期:2020-07-09
中文翻译:

解开铁单原子催化剂上甲烷的非氧化转化之谜。
在二氧化硅限制的单原子铁催化剂上甲烷的直接,非氧化转化是催化领域的一个里程碑式的发现,但是所提出的气相反应机理仍需讨论。在这里,我们通过计算建模和仿真报告了一种表面反应机理。甲烷的活化发生在单个铁位点,而在反应条件下,离解的甲基不利于解吸成气相。相比之下,离解的甲基更喜欢转移到活性中心的相邻碳位点(Fe 1 ©SiC 2),然后进行C-C偶联和氢转移,从而通过键-CH-CH 2生成主要产物(乙烯)。中间。我们发现了一种准Mars-van Krevelen(准MvK)表面反应机制,涉及提取和重新填充表面碳原子以实现甲烷在Fe 1 ©SiO 2上的非氧化转化,并且该表面过程被认为比其他气体更可信阶段反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号