当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
trans-Selective Aryldifluoroalkylation of Endocyclic Enecarbamates and Enamides by Nickel Catalysis.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-08 , DOI: 10.1002/anie.202008498 Chang Xu 1 , Ran Cheng 1 , Yun-Cheng Luo 1 , Ming-Kuan Wang 1 , Xingang Zhang 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-08 , DOI: 10.1002/anie.202008498 Chang Xu 1 , Ran Cheng 1 , Yun-Cheng Luo 1 , Ming-Kuan Wang 1 , Xingang Zhang 1, 2
Affiliation
|
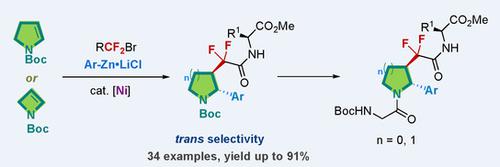
Efficient methods for the dicarbofuntionalization of the cyclic alkenes 2‐pyrroline and 2‐azetine are limited. Particularly, the dicarbofunctionalization of endocyclic enecarbamates to achieve fluorinated compounds remains an unsolved issue. Reported here is a nickel‐catalyzed trans‐selective dicarbofunctionalization of N‐Boc‐2‐pyrroline and N‐Boc‐2‐azetine, a class of endocyclic enecarbamates previously unexplored for transition metal catalyzed dicarbofunctionalization. The reaction can be extended to six‐ and seven‐membered endocyclic enamides. A variety of arylzinc reagents and bromodifluoroacetate, and its derivatives, undergo the reaction, providing straightforward and efficient access to an array of pyrrolidine‐ and azetidine‐containing fluorinated amino acids and oligopeptides, which may have applications in the life sciences.
中文翻译:

镍催化反式环内氨基甲酸酯和酰胺的芳基二氟烷基化反应。
限制环状烯烃2-吡咯啉和2-氮杂环丁烷二甲磺酰化的有效方法受到限制。特别地,内环烯氨基甲酸酯的二碳官能化以实现氟化化合物仍然是未解决的问题。这里报道的是N ‐ Boc ‐2‐吡咯啉和N的镍催化的反选择双碳官能化‐Boc‐2–氮杂环丁烷,一类以前尚未开发用于过渡金属催化双碳官能化的内环烯氨基甲酸酯。该反应可扩展至六元和七元环内酰胺。各种芳基锌试剂和溴代二氟乙酸盐及其衍生物进行反应,可直接有效地获得一系列含有吡咯烷和氮杂环丁烷的氟化氨基酸和寡肽,它们可能在生命科学中得到应用。
更新日期:2020-07-08
中文翻译:

镍催化反式环内氨基甲酸酯和酰胺的芳基二氟烷基化反应。
限制环状烯烃2-吡咯啉和2-氮杂环丁烷二甲磺酰化的有效方法受到限制。特别地,内环烯氨基甲酸酯的二碳官能化以实现氟化化合物仍然是未解决的问题。这里报道的是N ‐ Boc ‐2‐吡咯啉和N的镍催化的反选择双碳官能化‐Boc‐2–氮杂环丁烷,一类以前尚未开发用于过渡金属催化双碳官能化的内环烯氨基甲酸酯。该反应可扩展至六元和七元环内酰胺。各种芳基锌试剂和溴代二氟乙酸盐及其衍生物进行反应,可直接有效地获得一系列含有吡咯烷和氮杂环丁烷的氟化氨基酸和寡肽,它们可能在生命科学中得到应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号