Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-07-09 , DOI: 10.1016/j.molliq.2020.113773 Nnamdi C. Okey , Nnamdi L. Obasi , Paul M. Ejikeme , Derek T. Ndinteh , Ponnadurai Ramasami , El-Sayed M. Sherif , Ekemini D. Akpan , Eno E. Ebenso
|
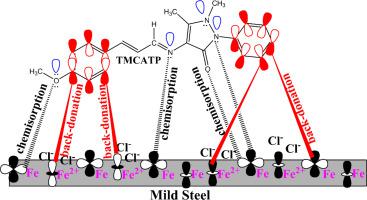
The corrosion inhibition of steel in 1 M HCl in the presence of four synthesized Schiff bases: 3-[(3-Hydroxy-4-methoxy-benzylidene)-amino]-benzoic acid (HMAMB), 3-[(2-Hydroxy-naphthalen-1-ylmethylene)-amino]-benzoic acid (HNCAMB), 3-[3-(4-Methoxy-phenyl)-allylideneamino]-benzoic acid (TMCAMB) and 4-[3-(4-Methoxy-phenyl)-allylideneamino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one (TMCATP) was studied by potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), surface analyses and computational studies. The inhibition efficiency of HMAMB, HNCAMB, TMCAMB and TMCATP obtained at the optimum inhibitor concentration (1.0 mM) from polarization measurement were 87.3%, 89.1%, 96.5% and 97.0% respectively. The values of inhibition efficiencies obtained via the electrochemical analyses for the four inhibitors were comparable. The data from polarization measurement implies the compounds inhibited as mixed-type inhibiting molecules in the tested media but mostly minimized the cathodic reaction. The data obtained experimentally fitted well into Langmuir adsorption isotherm with R2 values and slopes close to unity. The values of Gibbs free energy of adsorption ranged from −33.96 to −37.62 kJ mol−1, suggesting that the molecules adsorbed spontaneously to the surface of the metal by interchange of physical and chemical adsorption mechanisms. The energy dispersive X-ray (EDX) spectra results displayed a decrease in the percentage of oxygen on the surface of the mild steel and scanning electron microscopy (SEM) micrograph also indicated a minimal damage of the metal surface in solutions containing the Schiff bases when likened to the solution without the inhibitors. The results of DFT calculations showed that the molecules had high tendencies in interacting with the steel surface. The values of energy gap (eV) obtained were 4.09, 3.74, 3.69 and 3.58 while the estimated adsorption energy obtained from molecular dynamic simulation studies were − 654.2, −760.8, −741.3 and − 951.9 kJ mol−1 for HMAMB, HNCAMB, TMCAMB and TMCATP, respectively.
中文翻译:

某些氨基苯甲酸和4-氨基安替比林衍生的席夫碱作为酸性介质中低碳钢腐蚀抑制剂的评估:合成,实验和计算研究
在四种合成席夫碱的存在下,钢在1 M HCl中的腐蚀抑制作用:3-[(3-羟基-4-甲氧基-亚苄基)-氨基]-苯甲酸(HMAMB),3-[(2-羟基-萘-1-基亚甲基)-氨基]-苯甲酸(HNCAMB),3- [3-(4-甲氧基-苯基)-亚芳基氨基]-苯甲酸(TMCAMB)和4- [3-(4-甲氧基-苯基)通过电位动力学极化(PDP),电化学阻抗谱(EIS),表面分析和计算研究研究了-烯丙基亚氨基] -1,5-二甲基-2-苯基-1,2-二氢吡唑-3-酮(TMCATP)。通过极化测量在最佳抑制剂浓度(1.0 mM)下获得的HMAMB,HNCAMB,TMCAMB和TMCATP的抑制效率分别为87.3%,89.1%,96.5%和97.0%。通过获得的抑制效率值四种抑制剂的电化学分析具有可比性。极化测量的数据表明,在测试介质中这些化合物被抑制为混合型抑制分子,但大部分使阴极反应最小。实验获得的数据非常适合Langmuir吸附等温线,其R 2值和斜率接近于1。吉布斯吸附自由能的值介于-33.96至-37.62 kJ mol -1之间,表明分子通过物理和化学吸附机制的互换而自发地吸附到金属表面。能量色散X射线(EDX)光谱结果显示低碳钢表面上的氧气百分比降低,扫描电子显微镜(SEM)显微照片还表明,当含有席夫碱时,溶液中金属表面的损伤最小比喻为无抑制剂的溶液。DFT计算的结果表明,分子具有与钢表面相互作用的高趋势。获得的能隙(eV)值为4.09、3.74、3.69和3.58,而通过分子动力学模拟研究获得的估计吸附能为-654.2,-760.8,-741.3和-951.9 kJ mol -1 分别用于HMAMB,HNCAMB,TMCAMB和TMCATP。



























 京公网安备 11010802027423号
京公网安备 11010802027423号