Environmental Pollution ( IF 7.6 ) Pub Date : 2020-07-09 , DOI: 10.1016/j.envpol.2020.115189 Yalou Sun 1 , Duoqiang Pan 1 , Xiaoyan Wei 1 , Dongfan Xian 2 , Peng Wang 1 , Junjun Hou 1 , Zhen Xu 1 , Chunli Liu 2 , Wangsuo Wu 1
|
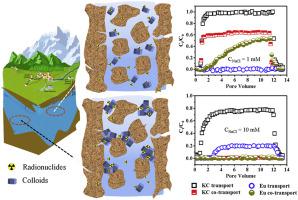
Environmental colloids play crucial roles in the transport of environmental pollutants in porous media by acting as pollutant carriers. In this work, the dispersion stability and correlated transport of kaolinite colloid were investigated as a function of solution pH, solution ionic strength, and concentration of humic acid (HA), the roles of kaolinite colloid in driving Eu(III) transport were discussed. The results showed that the dispersion of kaolinite colloid was favorable at alkaline and extremely acidic pH values, the trend of aggregation with varying pH was critically reversed at pH ∼3.2 due to the transformation of surface electrical properties. Cations with higher valence and mineral affinity showed a more significant contribution in inducing colloid aggregation, which was generally in accordance with the Schulze-Hardy rule and Hofmeister series. HA greatly increased the colloid stability by altering the surface electrostatic potential and steric effect. The Derjguin-Landau-Verwey-Overbeek (DLVO) model suggested that the electrostatic force between colloidal particles controlled the aggregation and destabilizing trend of colloid, and the theoretically calculated critical coagulation concentration was consistent with that determined from kinetic aggregation experiments. The roles of kaolinite colloid in driving Eu(III) transport varied under different conditions, and the transport behavior was highly correlated with the dispersion stability trend of colloid. These results can provide an enhanced understanding of the environmental fate of kaolinite colloid as well as commensal pollutants.
中文翻译:

洞察高岭石胶体的稳定性和相关运输:pH,电解质和腐殖质的影响。
环境胶体通过充当污染物载体,在多孔介质中环境污染物的运输中起着至关重要的作用。在这项工作中,研究了高岭石胶体的分散稳定性和相关迁移随溶液pH,溶液离子强度和腐殖酸(HA)浓度的变化,并讨论了高岭石胶体在驱动Eu(III)迁移中的作用。结果表明,高岭石胶体在碱性和极酸性pH值下均具有良好的分散性,由于表面电性能的转变,在pH〜3.2时,随着pH值的变化,聚集趋势发生了逆转。具有较高价和矿物质亲和力的阳离子在诱导胶体聚集方面表现出更重要的作用,通常符合Schulze-Hardy规则和Hofmeister系列。HA通过改变表面静电势和空间效应大大提高了胶体稳定性。Derjguin-Landau-Verwey-Overbeek(DLVO)模型表明,胶体颗粒之间的静电力控制了胶体的聚集和失稳趋势,并且理论计算的临界凝结浓度与动力学聚集实验确定的一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。HA通过改变表面静电势和空间效应大大提高了胶体稳定性。Derjguin-Landau-Verwey-Overbeek(DLVO)模型表明,胶体颗粒之间的静电力控制了胶体的聚集和失稳趋势,并且理论计算的临界凝结浓度与动力学聚集实验确定的一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。HA通过改变表面静电势和空间效应大大提高了胶体稳定性。Derjguin-Landau-Verwey-Overbeek(DLVO)模型表明,胶体颗粒之间的静电力控制了胶体的聚集和失稳趋势,并且理论计算的临界凝结浓度与动力学聚集实验确定的一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。Derjguin-Landau-Verwey-Overbeek(DLVO)模型表明,胶体颗粒之间的静电力控制了胶体的聚集和失稳趋势,并且理论计算的临界凝结浓度与动力学聚集实验确定的一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。Derjguin-Landau-Verwey-Overbeek(DLVO)模型表明,胶体颗粒之间的静电力控制了胶体的聚集和失稳趋势,并且理论计算的临界凝结浓度与动力学聚集实验确定的一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。理论计算得出的临界凝结浓度与动力学聚集实验确定的浓度一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。理论计算得出的临界凝结浓度与动力学聚集实验确定的浓度一致。高岭石胶体在驱动Eu(III)迁移中的作用在不同条件下有所不同,并且其迁移行为与胶体的分散稳定性趋势高度相关。这些结果可以提高对高岭石胶体以及常见污染物的环境命运的理解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号