Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-07-09 , DOI: 10.1016/j.chembiol.2020.06.015 Senlian Hong 1 , Lei Feng 2 , Yi Yang 1 , Hao Jiang 3 , Xiaomeng Hou 1 , Peng Guo 4 , Florence L Marlow 5 , Pamela Stanley 6 , Peng Wu 1
|
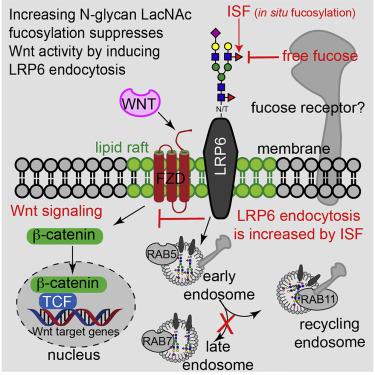
Wnt/β-catenin signaling regulates critical, context-dependent transcription in numerous physiological events. Among the well-documented mechanisms affecting Wnt/β-catenin activity, modification of N-glycans by L-fucose is the newest and the least understood. Using a combination of Chinese hamster ovary cell mutants with different fucosylation levels and cell-surface fucose editing (in situ fucosylation [ISF]), we report that α(1–3)-fucosylation of N-acetylglucosamine (GlcNAc) in the Galβ(1–4)-GlcNAc sequences of complex N-glycans modulates Wnt/β-catenin activity by regulating the endocytosis of low-density lipoprotein receptor-related protein 6 (LRP6). Pulse-chase experiments reveal that ISF elevates endocytosis of lipid-raft-localized LRP6, leading to the suppression of Wnt/β-catenin signaling. Remarkably, Wnt activity decreased by ISF is fully reversed by the exogenously added fucose. The combined data show that in situ cell-surface fucosylation can be exploited to regulate a specific signaling pathway via endocytosis promoted by a fucose-binding protein, thereby linking glycosylation of a receptor with its intracellular signaling.











































 京公网安备 11010802027423号
京公网安备 11010802027423号