当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the impact of the N3-substituted alkyl chain on the electronic environment of the cation and the anion for 1,3-dialkylimidazolium ionic liquids.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0cp02325f Shuang Men 1 , Yujuan Jin 2 , Peter Licence 3
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0cp02325f Shuang Men 1 , Yujuan Jin 2 , Peter Licence 3
Affiliation
|
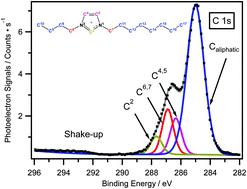
In this study, X-ray photoelectron spectroscopy is used to probe the impact of the N3-substituted alkyl group on the electronic environment of the cation and the anion by comparing two types of imidazolium cations, 1-alkyl-3-butylimidazolium and 1-alkyl-3-methylimidazolium. Due to the more intense inductive effect changing from methyl to butyl, the electronic environment of the cationic nitrogen can be significantly affected, which is reflected in a shift of N 1s binding energy. The magnitude of the binding energy shift is found to be more pronounced in the case of the less basic anion and inversely proportional to the basicity of the anion. The increase of the N3-substituted alkyl chain length can also influence the charge-transfer effect from the anion to the cation. This gives rise to a change in the electronic environment of the anion. Such an impact is found to be concentrated on the anion-based component bearing more negative point charges.
中文翻译:

探究N3取代的烷基链对1,3-二烷基咪唑鎓离子液体的阳离子和阴离子的电子环境的影响。
在这项研究中,通过比较两种咪唑阳离子,即1-烷基-3-丁基咪唑鎓和1-咪唑鎓阳离子,使用X射线光电子能谱探测N3取代的烷基对阳离子和阴离子的电子环境的影响。烷基-3-甲基咪唑鎓。由于从甲基到丁基的更强烈的感应效应,可显着影响阳离子氮的电子环境,这反映在N 1s结合能的转移上。发现在碱性较低的阴离子的情况下,结合能移动的幅度更加明显,并且与阴离子的碱性成反比。N 3-取代的烷基链长度的增加也可以影响从阴离子到阳离子的电荷转移效果。这引起阴离子电子环境的变化。
更新日期:2020-08-05
中文翻译:

探究N3取代的烷基链对1,3-二烷基咪唑鎓离子液体的阳离子和阴离子的电子环境的影响。
在这项研究中,通过比较两种咪唑阳离子,即1-烷基-3-丁基咪唑鎓和1-咪唑鎓阳离子,使用X射线光电子能谱探测N3取代的烷基对阳离子和阴离子的电子环境的影响。烷基-3-甲基咪唑鎓。由于从甲基到丁基的更强烈的感应效应,可显着影响阳离子氮的电子环境,这反映在N 1s结合能的转移上。发现在碱性较低的阴离子的情况下,结合能移动的幅度更加明显,并且与阴离子的碱性成反比。N 3-取代的烷基链长度的增加也可以影响从阴离子到阳离子的电荷转移效果。这引起阴离子电子环境的变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号