当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding alcohol aggregates and the water hydrogen bond network towards miscibility in alcohol solutions: graph theoretical analysis.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0cp01991g Seungeui Choi 1 , Saravanan Parameswaran , Jun-Ho Choi
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0cp01991g Seungeui Choi 1 , Saravanan Parameswaran , Jun-Ho Choi
Affiliation
|
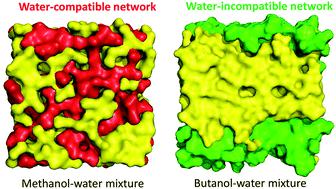
Under ambient conditions, methanol and ethanol are miscible in water at all concentrations, while n-butanol is partially miscible. This is the first study to quantitatively examine the miscibility of butanol and compare with miscible alcohols by employing molecular dynamics simulations and graph theoretical analysis of three water-alcohol mixtures at various concentrations. We show how distinct alcohol aggregates are formed, thereby affecting the water structure, which established the relationship between the morphological structure of the aggregates and the miscibility of the alcohol in aqueous solution. The aggregates of methanol and ethanol in highly concentrated solutions form an extended H-bond network that intertwines well with the H-bond network of water. n-Butanol tends to self-associate and form large aggregates, while such aggregates are segregated from water. Graph theoretical analysis revealed that the alcohol aggregates of methanol and ethanol solutions have a morphological structure different from that of n-butanol, although there is no significant difference in morphology between the three pure alcohols. These two distinct alcohol aggregates are classified as water-compatible and water-incompatible depending upon their interaction with the water H-bond network, and their effect on the water structure was investigated. Our study reveals that the water-compatible network of alcohol aggregates in methanol and ethanol solutions disrupts the water H-bond networks, while the water-incompatible network of n-butanol aggregates does not considerably alter the water structure, which is consistent with the experimental results. Furthermore, we propose that miscible alcohols form water-compatible networks in binary aqueous systems while partially miscible alcohols form water-incompatible networks. The bifurcating hypothesis on the alcohol aggregation behavior in liquid water is of critical use to understand the fundamental issues such as solubility and phase separation in solution systems.
中文翻译:

理解醇聚集体和水氢键网络与醇溶液的互溶性:图形理论分析。
在环境条件下,甲醇和乙醇在所有浓度下均可与水混溶,而正丁醇可部分混溶。这是第一项定量研究丁醇的可混溶性并通过分子动力学模拟和图形理论分析三种不同浓度的水-醇混合物与可混溶的醇进行比较的研究。我们展示了如何形成独特的醇聚集体,从而影响水的结构,从而建立了聚集体的形态结构与醇在水溶液中的混溶性之间的关系。高浓度溶液中甲醇和乙醇的聚集体形成扩展的氢键网络,该氢键网络与水的氢键网络交织得很好。ñ-丁醇倾向于自缔合并形成大的聚集体,而这种聚集体与水分离。图理论分析显示甲醇和乙醇溶液的醇聚集体具有与正丁醇不同的形态结构,尽管三种纯醇之间的形态没有显着差异。根据这两种截然不同的醇聚集体与水H键网络的相互作用,将其分为水相容性和水不相容性,并研究了它们对水结构的影响。我们的研究表明,甲醇和乙醇溶液中酒精聚集体的水相容网络破坏了水的H键网络,而n的水不相容网络-丁醇聚集体不会显着改变水的结构,这与实验结果一致。此外,我们提出可混溶的醇在二元水性体系中形成水相容性网络,而部分可混溶的醇形成水不相容性网络。对于了解液体系统中的溶解度和相分离等基本问题,液态水中醇聚集行为的分叉假设至关重要。
更新日期:2020-08-05
中文翻译:

理解醇聚集体和水氢键网络与醇溶液的互溶性:图形理论分析。
在环境条件下,甲醇和乙醇在所有浓度下均可与水混溶,而正丁醇可部分混溶。这是第一项定量研究丁醇的可混溶性并通过分子动力学模拟和图形理论分析三种不同浓度的水-醇混合物与可混溶的醇进行比较的研究。我们展示了如何形成独特的醇聚集体,从而影响水的结构,从而建立了聚集体的形态结构与醇在水溶液中的混溶性之间的关系。高浓度溶液中甲醇和乙醇的聚集体形成扩展的氢键网络,该氢键网络与水的氢键网络交织得很好。ñ-丁醇倾向于自缔合并形成大的聚集体,而这种聚集体与水分离。图理论分析显示甲醇和乙醇溶液的醇聚集体具有与正丁醇不同的形态结构,尽管三种纯醇之间的形态没有显着差异。根据这两种截然不同的醇聚集体与水H键网络的相互作用,将其分为水相容性和水不相容性,并研究了它们对水结构的影响。我们的研究表明,甲醇和乙醇溶液中酒精聚集体的水相容网络破坏了水的H键网络,而n的水不相容网络-丁醇聚集体不会显着改变水的结构,这与实验结果一致。此外,我们提出可混溶的醇在二元水性体系中形成水相容性网络,而部分可混溶的醇形成水不相容性网络。对于了解液体系统中的溶解度和相分离等基本问题,液态水中醇聚集行为的分叉假设至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号