当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Haloboration: scope, mechanism and utility
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0nj02908d Sven Kirschner 1 , Kang Yuan 1 , Michael J Ingleson 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0nj02908d Sven Kirschner 1 , Kang Yuan 1 , Michael J Ingleson 1
Affiliation
|
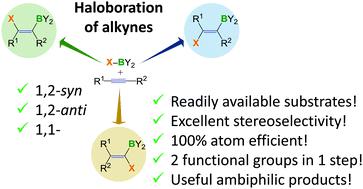
Haloboration, the addition of B–X (X = Cl, Br, I) across an unsaturated moiety e.g., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y or C
Y or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Y (Y = C, N, etc.), is dramatically less utilised than the ubiquitous hydroboration reaction. However, haloboration of alkynes in particular is a useful tool to access ambiphilic 1,2-disubstituted alkenes. The stereochemical outcome of the reaction is easily controlled and the resulting products have proven to be valuable building blocks in organic synthesis and materials chemistry. This review aims at providing the reader with a brief summary of the historic development and of the current mechanistic understanding of this transformation. Recent developments are discussed and select examples demonstrating the use of haloboration products are given with a focus on the major areas, specifically, natural product synthesis and the development of boron-doped polycyclic aromatic hydrocarbons (B-PAHs).
Y (Y = C, N, etc.), is dramatically less utilised than the ubiquitous hydroboration reaction. However, haloboration of alkynes in particular is a useful tool to access ambiphilic 1,2-disubstituted alkenes. The stereochemical outcome of the reaction is easily controlled and the resulting products have proven to be valuable building blocks in organic synthesis and materials chemistry. This review aims at providing the reader with a brief summary of the historic development and of the current mechanistic understanding of this transformation. Recent developments are discussed and select examples demonstrating the use of haloboration products are given with a focus on the major areas, specifically, natural product synthesis and the development of boron-doped polycyclic aromatic hydrocarbons (B-PAHs).
中文翻译:

Haloboration:范围、机制和效用
卤化,在不饱和部分例如C![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y 或 C
Y 或 C ![[三键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Y(Y = C、N等)上添加 B–X(X = Cl、Br、I)。),比普遍存在的硼氢化反应要少得多。然而,炔烃的卤化尤其是获得两亲性 1,2-二取代烯烃的有用工具。反应的立体化学结果很容易控制,所得产物已被证明是有机合成和材料化学中有价值的构建模块。这篇综述旨在向读者简要总结历史发展和目前对这种转变的机制理解。讨论了最近的发展,并选择了展示卤化产品使用的例子,重点放在主要领域,特别是天然产物合成和掺硼多环芳烃 (B-PAHs) 的开发。
Y(Y = C、N等)上添加 B–X(X = Cl、Br、I)。),比普遍存在的硼氢化反应要少得多。然而,炔烃的卤化尤其是获得两亲性 1,2-二取代烯烃的有用工具。反应的立体化学结果很容易控制,所得产物已被证明是有机合成和材料化学中有价值的构建模块。这篇综述旨在向读者简要总结历史发展和目前对这种转变的机制理解。讨论了最近的发展,并选择了展示卤化产品使用的例子,重点放在主要领域,特别是天然产物合成和掺硼多环芳烃 (B-PAHs) 的开发。
更新日期:2020-07-15
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y or C
Y or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Y (Y = C, N, etc.), is dramatically less utilised than the ubiquitous hydroboration reaction. However, haloboration of alkynes in particular is a useful tool to access ambiphilic 1,2-disubstituted alkenes. The stereochemical outcome of the reaction is easily controlled and the resulting products have proven to be valuable building blocks in organic synthesis and materials chemistry. This review aims at providing the reader with a brief summary of the historic development and of the current mechanistic understanding of this transformation. Recent developments are discussed and select examples demonstrating the use of haloboration products are given with a focus on the major areas, specifically, natural product synthesis and the development of boron-doped polycyclic aromatic hydrocarbons (B-PAHs).
Y (Y = C, N, etc.), is dramatically less utilised than the ubiquitous hydroboration reaction. However, haloboration of alkynes in particular is a useful tool to access ambiphilic 1,2-disubstituted alkenes. The stereochemical outcome of the reaction is easily controlled and the resulting products have proven to be valuable building blocks in organic synthesis and materials chemistry. This review aims at providing the reader with a brief summary of the historic development and of the current mechanistic understanding of this transformation. Recent developments are discussed and select examples demonstrating the use of haloboration products are given with a focus on the major areas, specifically, natural product synthesis and the development of boron-doped polycyclic aromatic hydrocarbons (B-PAHs).
中文翻译:

Haloboration:范围、机制和效用
卤化,在不饱和部分例如C
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y 或 C
Y 或 C ![[三键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Y(Y = C、N等)上添加 B–X(X = Cl、Br、I)。),比普遍存在的硼氢化反应要少得多。然而,炔烃的卤化尤其是获得两亲性 1,2-二取代烯烃的有用工具。反应的立体化学结果很容易控制,所得产物已被证明是有机合成和材料化学中有价值的构建模块。这篇综述旨在向读者简要总结历史发展和目前对这种转变的机制理解。讨论了最近的发展,并选择了展示卤化产品使用的例子,重点放在主要领域,特别是天然产物合成和掺硼多环芳烃 (B-PAHs) 的开发。
Y(Y = C、N等)上添加 B–X(X = Cl、Br、I)。),比普遍存在的硼氢化反应要少得多。然而,炔烃的卤化尤其是获得两亲性 1,2-二取代烯烃的有用工具。反应的立体化学结果很容易控制,所得产物已被证明是有机合成和材料化学中有价值的构建模块。这篇综述旨在向读者简要总结历史发展和目前对这种转变的机制理解。讨论了最近的发展,并选择了展示卤化产品使用的例子,重点放在主要领域,特别是天然产物合成和掺硼多环芳烃 (B-PAHs) 的开发。









































 京公网安备 11010802027423号
京公网安备 11010802027423号