当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anticancer potential of some imidazole and fused imidazole derivatives: exploring the mechanism via epidermal growth factor receptor (EGFR) inhibition
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0md00146e Sourav Kalra 1 , Gaurav Joshi 2 , Manvendra Kumar 2 , Sahil Arora 2 , Harsimrat Kaur 3 , Sandeep Singh 1 , Anjana Munshi 1 , Raj Kumar 2
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0md00146e Sourav Kalra 1 , Gaurav Joshi 2 , Manvendra Kumar 2 , Sahil Arora 2 , Harsimrat Kaur 3 , Sandeep Singh 1 , Anjana Munshi 1 , Raj Kumar 2
Affiliation
|
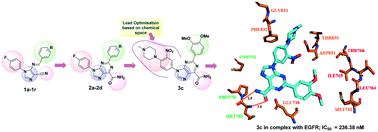
Imidazole-based epidermal growth factor receptor (EGFR) inhibitors were computationally designed and synthesized. All the compounds were assessed for their anti-proliferative activity against five cancer cell lines, viz., MDA-MB-231 (breast), T47D (breast) and MCF-7 (breast), A549 (lung) and HT-29 (colorectal). Compounds 2c and 2d emerged as better anticancer molecules with no toxicity towards normal cells. 2c and 2d inhibited EGFR enzymatic activity in vitro with IC50 values of 617.33 ± 0.04 nM and 710 ± 0.05 nM, respectively. In order to further improve the potency, we explored an unoccupied area of the ATP binding domain of EGFR and analysed an in silico interaction model of 2c and 2d–EGFR complexes that guided and allowed substitution of the 4-fluorophenyl ring (2c and 2d) with 4-(4-methylpiperazinyl)-3-nitrophenyl at the N-9 position, resulting in compound 3c with a better binding score and potent EGFR inhibitory activity (IC50: 236.38 ± 0.04 nM), which was comparable to the positive control erlotinib (239.91 ± 0.05 nM). 3c exhibited a great improvement in anticancer potency with inhibition of cell growth of all cancer cell lines at very low micromolar concentrations (IC50 = 1.98 to 4.07 μM). Further investigation revealed that 3c also induced an increase in ROS levels in cancer cells in a mitochondrial-independent manner and halted the cell cycle at the sub-G1 phase.
中文翻译:

一些咪唑和融合咪唑衍生物的抗癌潜力:通过抑制表皮生长因子受体(EGFR)探索机制
基于咪唑的表皮生长因子受体 (EGFR) 抑制剂是通过计算设计和合成的。评估了所有化合物对五种癌细胞系的抗增殖活性,即。、MDA-MB-231(乳房)、T47D(乳房)和 MCF-7(乳房)、A549(肺)和 HT-29(结肠直肠)。化合物2c和2d成为更好的抗癌分子,对正常细胞没有毒性。2c和2d在体外抑制EGFR酶活性,IC 50值分别为617.33 ± 0.04 nM和710 ± 0.05 nM。为了进一步提高效力,我们探索了EGFR的ATP结合域的一个未被占用的区域并对其进行了分析2c和2d -EGFR 配合物的计算机相互作用模型,该模型引导并允许在 N-9 位置用 4-(4-甲基哌嗪基)-3-硝基苯基取代 4-氟苯基环(2c和2d ),得到化合物3c具有更好的结合评分和有效的 EGFR 抑制活性 (IC 50 : 236.38 ± 0.04 nM),与阳性对照厄洛替尼 (239.91 ± 0.05 nM) 相当。图3c在极低的微摩尔浓度下(IC 50 = 1.98 至 4.07 μM)显示出抗癌效力的显着改善,抑制了所有癌细胞系的细胞生长。进一步调查显示,3c还以不依赖线粒体的方式诱导癌细胞中 ROS 水平的增加,并在 sub-G 1期停止细胞周期。
更新日期:2020-08-20
中文翻译:

一些咪唑和融合咪唑衍生物的抗癌潜力:通过抑制表皮生长因子受体(EGFR)探索机制
基于咪唑的表皮生长因子受体 (EGFR) 抑制剂是通过计算设计和合成的。评估了所有化合物对五种癌细胞系的抗增殖活性,即。、MDA-MB-231(乳房)、T47D(乳房)和 MCF-7(乳房)、A549(肺)和 HT-29(结肠直肠)。化合物2c和2d成为更好的抗癌分子,对正常细胞没有毒性。2c和2d在体外抑制EGFR酶活性,IC 50值分别为617.33 ± 0.04 nM和710 ± 0.05 nM。为了进一步提高效力,我们探索了EGFR的ATP结合域的一个未被占用的区域并对其进行了分析2c和2d -EGFR 配合物的计算机相互作用模型,该模型引导并允许在 N-9 位置用 4-(4-甲基哌嗪基)-3-硝基苯基取代 4-氟苯基环(2c和2d ),得到化合物3c具有更好的结合评分和有效的 EGFR 抑制活性 (IC 50 : 236.38 ± 0.04 nM),与阳性对照厄洛替尼 (239.91 ± 0.05 nM) 相当。图3c在极低的微摩尔浓度下(IC 50 = 1.98 至 4.07 μM)显示出抗癌效力的显着改善,抑制了所有癌细胞系的细胞生长。进一步调查显示,3c还以不依赖线粒体的方式诱导癌细胞中 ROS 水平的增加,并在 sub-G 1期停止细胞周期。



























 京公网安备 11010802027423号
京公网安备 11010802027423号