当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst control of selectivity in the C–O bond alumination of biomass derived furans
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0sc01918f Thomas N. Hooper 1, 2, 3, 4, 5 , Ryan K. Brown 1, 2, 3, 4, 5 , Feriel Rekhroukh 1, 2, 3, 4, 5 , Martí Garçon 1, 2, 3, 4, 5 , Andrew J. P. White 1, 2, 3, 4, 5 , Paulo J. Costa 6, 7, 8, 9, 10 , Mark R. Crimmin 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-08 , DOI: 10.1039/d0sc01918f Thomas N. Hooper 1, 2, 3, 4, 5 , Ryan K. Brown 1, 2, 3, 4, 5 , Feriel Rekhroukh 1, 2, 3, 4, 5 , Martí Garçon 1, 2, 3, 4, 5 , Andrew J. P. White 1, 2, 3, 4, 5 , Paulo J. Costa 6, 7, 8, 9, 10 , Mark R. Crimmin 1, 2, 3, 4, 5
Affiliation
|
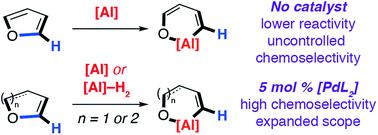
Non-catalysed and catalysed reactions of aluminium reagents with furans, dihydrofurans and dihydropyrans were investigated and lead to ring-expanded products due to the insertion of the aluminium reagent into a C–O bond of the heterocycle. Specifically, the reaction of [{(ArNCMe)2CH}Al] (Ar = 2,6-di-iso-propylphenyl, 1) with furans proceeded between 25 and 80 °C leading to dearomatised products due to the net transformation of a sp2 C–O bond into a sp2 C–Al bond. The kinetics of the reaction of 1 with furan were found to be 1st order with respect to 1 with activation parameters ΔH‡ = +19.7 (±2.7) kcal mol−1, ΔS‡ = −18.8 (±7.8) cal K−1 mol−1 and ΔG‡298 K = +25.3 (±0.5) kcal mol−1 and a KIE of 1.0 ± 0.1. DFT calculations support a stepwise mechanism involving an initial (4 + 1) cycloaddition of 1 with furan to form a bicyclic intermediate that rearranges by an α-migration. The selectivity of ring-expansion is influenced by factors that weaken the sp2 C–O bond through population of the σ*-orbital. Inclusion of [Pd(PCy3)2] as a catalyst in these reactions results in expansion of the substrate scope to include 2,3-dihydrofurans and 3,4-dihydropyrans and improves selectivity. Under catalysed conditions, the C–O bond that breaks is that adjacent to the sp2C–H bond. The aluminium(III) dihydride reagent [{(MesNCMe)2CH}AlH2] (Mes = 2,4,6-trimethylphenyl, 2) can also be used under catalytic conditions to effect a dehydrogenative ring-expansion of furans. Further mechanistic analysis shows that C–O bond functionalisation occurs via an initial C–H bond alumination. Kinetic products can be isolated that are derived from installation of the aluminium reagent at the 2-position of the heterocycle. C–H alumination occurs with a KIE of 4.8 ± 0.3 consistent with a turnover limiting step involving oxidative addition of the C–H bond to the palladium catalyst. Isomerisation of the kinetic C–H aluminated product to the thermodynamic C–O ring expansion product is an intramolecular process that is again catalysed by [Pd(PCy3)2]. DFT calculations suggest that the key C–O bond breaking step involves attack of an aluminium based metalloligand on the 2-palladated heterocycle. The new methodology has been applied to important platform chemicals from biomass.
中文翻译:

生物质衍生呋喃的C–O键铝化反应中催化剂的选择性控制
对铝试剂与呋喃,二氢呋喃和二氢吡喃的非催化和催化反应进行了研究,由于将铝试剂插入杂环的C-O键中,导致生成环扩产物。具体而言,[{((ArNCMe)2 CH} Al](Ar = 2,6-二异丙基苯基,1)与呋喃的反应在25至80°C之间进行,这是由于a的净转化而导致的脱芳构产物将sp 2 C–O键转换为sp 2 C–Al键。发现1与呋喃的反应动力学相对于1具有活化参数ΔH ‡ = +19.7(±2.7)kcal mol -1,Δ为1阶。S ‡ = -18.8(±7.8)cal K -1 mol -1和ΔG ‡ 298 K = +25.3(±0.5)kcal mol -1和KIE为1.0±0.1。DFT计算支持一种逐步机制,该机制涉及将1与呋喃进行初始(4 + 1)环加成反应,形成一个通过α迁移重新排列的双环中间体。扩环的选择性受因子的影响,这些因子会通过σ*轨道的填充减弱sp 2 C–O键。包含[Pd(PCy 3)2在这些反应中作为催化剂的反应导致底物范围扩大到包括2,3-二氢呋喃和3,4-二氢吡喃,并提高了选择性。在催化条件下,断裂的C–O键是与sp 2 C–H键相邻的键。二氢铝(III)试剂[{(MesNCMe)2 CH} AlH 2 ](Mes = 2,4,6-三甲基苯基,2)也可以在催化条件下用于使呋喃脱氢扩环。进一步的机理分析表明,C–O键功能化是通过最初的C–H键铝化。可以分离出动力学产物,该动力学产物源于将铝试剂安装在杂环的2位上。C–H发生铝化,其KIE为4.8±0.3,这与周转限制步骤有关,该步骤涉及将C–H键氧化加成到钯催化剂上。动力学C–H铝化产物向热力学C–O环膨胀产物的异构化是一个分子内过程,该过程再次被[Pd(PCy 3)2 ]催化。DFT计算表明,关键的C–O键断裂步骤涉及铝基金属配体对2-palladed杂环的攻击。新方法已应用于生物质中重要的平台化学品。
更新日期:2020-08-05
中文翻译:

生物质衍生呋喃的C–O键铝化反应中催化剂的选择性控制
对铝试剂与呋喃,二氢呋喃和二氢吡喃的非催化和催化反应进行了研究,由于将铝试剂插入杂环的C-O键中,导致生成环扩产物。具体而言,[{((ArNCMe)2 CH} Al](Ar = 2,6-二异丙基苯基,1)与呋喃的反应在25至80°C之间进行,这是由于a的净转化而导致的脱芳构产物将sp 2 C–O键转换为sp 2 C–Al键。发现1与呋喃的反应动力学相对于1具有活化参数ΔH ‡ = +19.7(±2.7)kcal mol -1,Δ为1阶。S ‡ = -18.8(±7.8)cal K -1 mol -1和ΔG ‡ 298 K = +25.3(±0.5)kcal mol -1和KIE为1.0±0.1。DFT计算支持一种逐步机制,该机制涉及将1与呋喃进行初始(4 + 1)环加成反应,形成一个通过α迁移重新排列的双环中间体。扩环的选择性受因子的影响,这些因子会通过σ*轨道的填充减弱sp 2 C–O键。包含[Pd(PCy 3)2在这些反应中作为催化剂的反应导致底物范围扩大到包括2,3-二氢呋喃和3,4-二氢吡喃,并提高了选择性。在催化条件下,断裂的C–O键是与sp 2 C–H键相邻的键。二氢铝(III)试剂[{(MesNCMe)2 CH} AlH 2 ](Mes = 2,4,6-三甲基苯基,2)也可以在催化条件下用于使呋喃脱氢扩环。进一步的机理分析表明,C–O键功能化是通过最初的C–H键铝化。可以分离出动力学产物,该动力学产物源于将铝试剂安装在杂环的2位上。C–H发生铝化,其KIE为4.8±0.3,这与周转限制步骤有关,该步骤涉及将C–H键氧化加成到钯催化剂上。动力学C–H铝化产物向热力学C–O环膨胀产物的异构化是一个分子内过程,该过程再次被[Pd(PCy 3)2 ]催化。DFT计算表明,关键的C–O键断裂步骤涉及铝基金属配体对2-palladed杂环的攻击。新方法已应用于生物质中重要的平台化学品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号